2016-2017 Influenza Season Week 2 ending January 14, 2017
January 21st, 2017During week 2 (January 8-14, 2017), influenza activity increased in the United States.
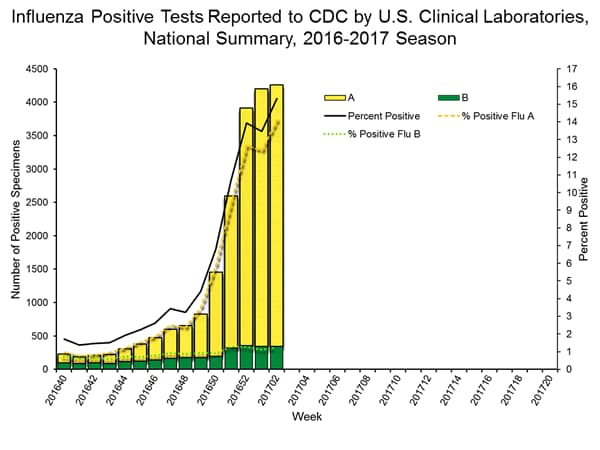
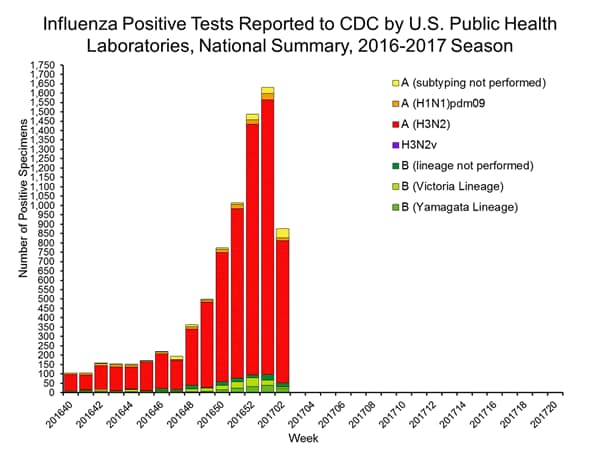
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 2 was influenza A (H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
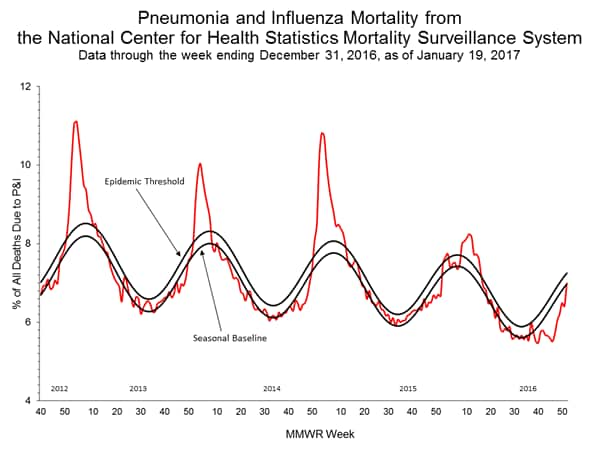
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
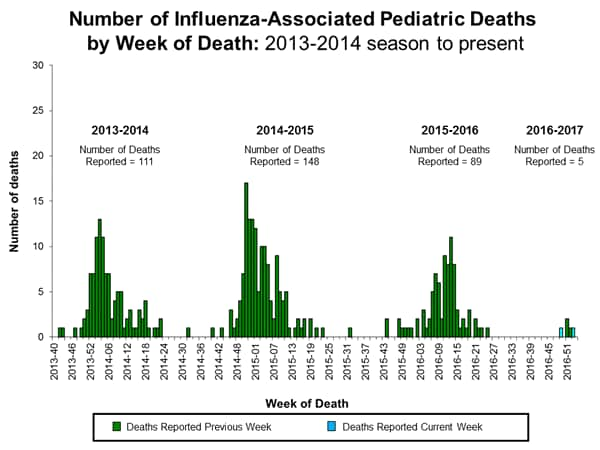
- Influenza-associated Pediatric Deaths: Two influenza-associated pediatric deaths were reported.
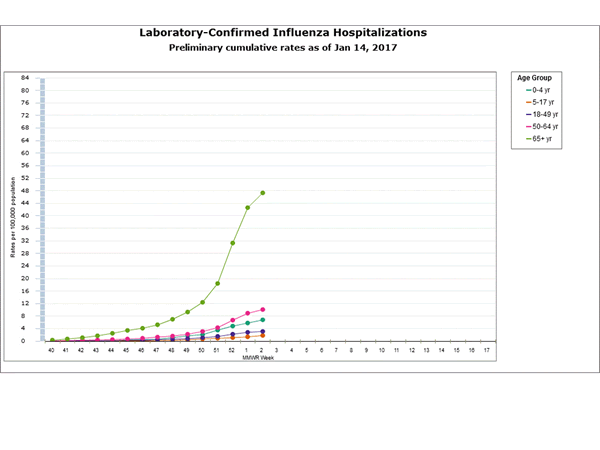
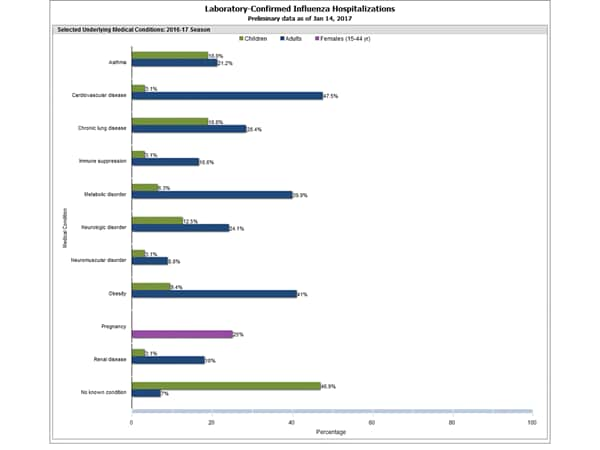
- Influenza-associated Hospitalizations: A cumulative rate for the season of 10.2 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
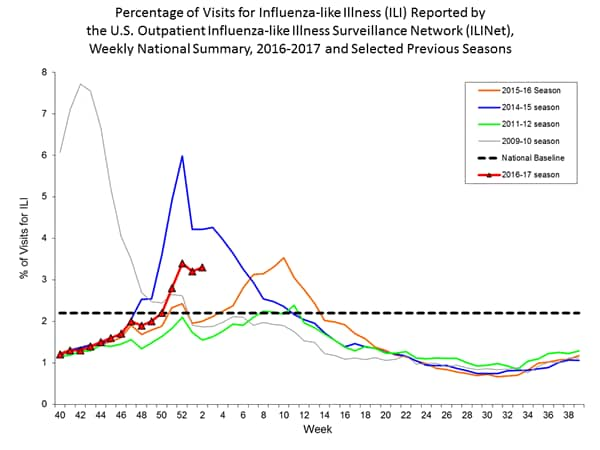
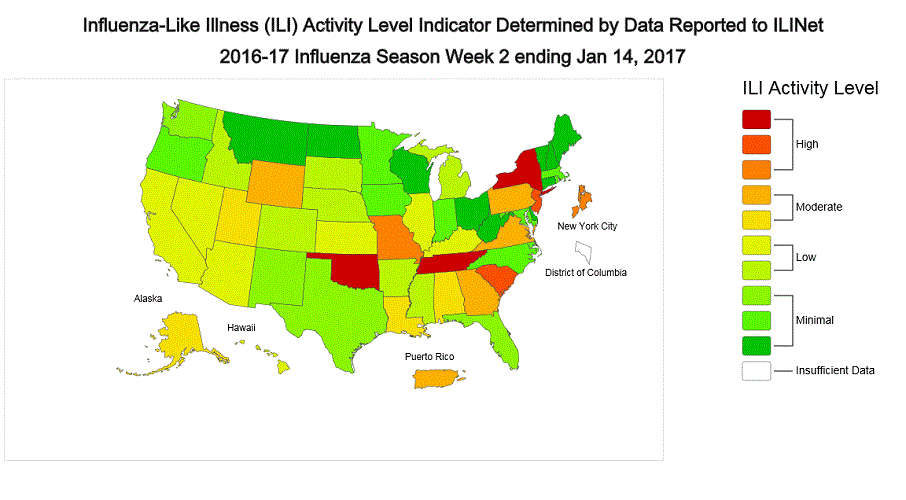
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 3.3%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above their region-specific baseline levels. New York City and six states experienced high ILI activity; Puerto Rico and eight states experienced moderate ILI activity; 14 states experienced low ILI activity; 22 states experienced minimal ILI activity, and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in Puerto Rico and 29 states was reported as widespread; Guam and 17 states reported regional activity; the District of Columbia and four states reported local activity; and the U.S. Virgin Islands reported sporadic activity.
Neuraminidase Inhibitor Resistance Testing Results on Samples Collected Since October 1, 2016
|
Oseltamivir |
Zanamivir |
Peramivir |
||||
|---|---|---|---|---|---|---|
|
Virus Samples tested (n) |
Resistant Viruses, Number (%) |
Virus Samples tested (n) |
Resistant Viruses, Number (%) |
Virus Samples tested (n) |
Resistant Viruses, Number (%) |
|
| Influenza A (H1N1)pdm09 |
59 |
0 (0.0) |
59 |
0 (0.0) |
59 |
0 (0.0) |
| Influenza A (H3N2) |
385 |
0 (0.0) |
385 |
0 (0.0) |
319 |
0 (0.0) |
| Influenza B |
101 |
0 (0.0) |
101 |
0 (0.0) |
101 |
0 (0.0) |
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A (H1N1)pdm09 viruses and oseltamivir-resistant influenza A (H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.