Archive for the ‘Dengue’ Category
Aedes albopictus, a vector for Zika, Dengue, and Yellow Fever, has been spotted in Eindhoven, Netherlands
Friday, October 5th, 2018The mosquito, Aedes albopictus, that is responsible for transmitting Zika virus and yellow fever is closing in on Portugal
Friday, October 5th, 2018Dengue in pregnancy may increase risk of congenital brain malformations
Tuesday, July 24th, 2018“….Researchers found that confirmed dengue during pregnancy increased the odds of a neurologic congenital anomaly by 50%, but the results were not statistically significant (95% confidence interval, 0.97-2.27). Congenital malformation of the spinal cord, however, and some congenital brain malformations were more than four times more frequent in dengue-affected births….”
Paixão ES, Teixeira MG, da Conceição N. Costa M, Barreto ML, Rodrigues LC. Enny S. Paixão. Symptomatic dengue during pregnancy and congenital neurologic malformations. Emerg Infect Dis. 2018 Sep [date cited]. https://doi.org/10.3201/eid2409.170361
Sterile Insect Technique on Aedes aegypti mosquitoes
Monday, July 16th, 2018In an international partnership between CSIRO, Verily and James Cook University, scientists used specialised technology to release millions of sterilised male Aedes aegypti mosquitoes across the Cassowary Coast in Queensland in a bid to combat the global pest.
CSIRO Director of Health and Biosecurity Dr Rob Grenfell said the results were a major win in the fight against diseases-spreading mosquitoes.
“The invasive Aedes aegypti mosquito is one of the world’s most dangerous pests, capable of spreading devastating diseases like dengue, Zika and chikungunya and responsible for infecting millions of people with disease around the world each year,” Dr Grenfell said.
“Increased urbanisation and warming temperatures mean that more people are at risk, as these mosquitoes which were once relegated to areas near the equator forge past previous climatic boundaries.
“Although the majority of mosquitoes don’t spread diseases, the three mostly deadly types the Aedes, Anopheles and Culex are found almost all over the world and are responsible for around 17 per cent of infectious disease transmissions globally.”
From November 2017 to June this year, non-biting male Aedes aegypti mosquitoes sterilised with the natural bacteria Wolbachia were released in trial zones along the Cassowary Coast in North Queensland.
They mated with local female mosquitoes, resulting in eggs that did not hatch and a significant reduction of their population.
“Our heartfelt thanks goes out to the Innisfail community who literally opened their doors to our team, letting us install mosquito traps around their homes and businesses – we couldn’t have done this without your support,” Dr Grenfell said.
The process, known as the Sterile Insect Technique, has been successfully used since the 1950s but the challenge in making it work for mosquitoes like the Aedes aegypti has been rearing enough mosquitoes, removing biting females, identifying the males and then releasing the huge numbers needed to suppress a population.
To address this challenge, Verily, an affiliate of Alphabet Inc, developed a mosquito rearing and sex sorting and release technology as part of its global Debug project.
“We’re very pleased to see strong suppression of these dangerous biting female Aedes aegypti mosquitoes,” Verily’s Nigel Snoad said.
“We are particularly thankful to the people of Innisfail for their strong support, which has been incredible.
“We came to Innisfail with CSIRO and JCU to see how this approach worked in a tropical environment where these mosquitoes thrive, and to learn what it was like to operate our technology with research collaborators as we work together to find new ways to tackle these dangerous mosquitoes.”
Scientists compared the number of Aedes aegypti mosquitoes trapped in release sites and control zones to monitor and track populations.
The millions of mosquitoes needed for the trial were reared at James Cook University in Cairns.
To produce the three million male mosquitoes needed for the trial, researchers at James Cook University (JCU) in Cairns set out to raise almost 20 million Aedes aegypti.
“We allowed for the possibility of deaths during the process, as well as the need to sift out the female half of the population,” Dr Kyran Staunton from James Cook University said.
“Verily’s technology enabled us to do the sex sorting faster and with much higher accuracy.
“We learnt a lot from collaborating on this first tropical trial and we’re excited to see how this approach might be applied in other regions where Aedes aegypti poses a threat to life and health.”
“The health of our nation is paramount as we help Australia achieve its vision to become one of the healthiest nations on earth,” CSIRO Chief Executive Dr Larry Marshall said.
“By enabling industry partners like Verily to leverage the world-leading health capability we have built in CSIRO we can deliver this moonshot and tackle some of the world’s most wicked challenges with science.”
Clinical differences between Dengue and Zika
Thursday, June 28th, 2018Yan G, Pang L, Cook AR, Ho HJ, Win MS, Khoo AL, et al. Distinguishing Zika and dengue viruses through simple clinical assessment, Singapore. Emerg Infect Dis. 2018 Aug [date cited]. https://doi.org/10.3201/eid2408.171883
“…Conjunctivitis strongly indicated Zika virus infection (odds ratio [OR] 30.1, 95% CI 9.57–94.44; p < 0.001). In contrast, fever (OR 0.05, 95% CI 0.01–0.47; p = 0.008), myalgia (OR 0.20, 95% CI 0.08–0.48; p<0.001), and headache (OR 0.12, 95% CI 0.05–0.30; p<0.001) were more prominent in patients with DENV infection.
Further, DENV patients tended to have thrombocytopenia (median platelet count 132 × 109/µL, range 15–386 × 109/µL) and monocytosis (median monocyte count 0.50 × 109/µL, range 0.11–1.70 × 109/µL), whereas Zika patients tended to have normal platelet (median 225 × 109/µL, range 128–326 × 109/µL; p<0.001) and monocyte (median 0.35 × 109/µL, range 0.13–1.00 × 109/µL; p = 0.021) counts……”
Climate change and dengue fever in Latin America
Monday, June 4th, 2018Felipe J. Colón-González el al., “Limiting global-mean temperature increase to 1.5–2 °C could reduce the incidence and spatial spread of dengue fever in Latin America,” PNAS (2018).
Read more at: https://phys.org/news/2018-05-limiting-global-millions-dengue-fever.html#jCp
“……We show that policies to limit global warming to 2 °C could reduce dengue cases by about 2.8 (0.8–7.4) million cases per year by the end of the century compared with a no-policy scenario that warms by 3.7 °C. Limiting warming further to 1.5 °C produces an additional drop in cases of about 0.5 (0.2–1.1) million per year…..”
Since January, Reunion has recorded 1,816 cases of Dengue Fever
Thursday, May 3rd, 2018On 16 March 2018, WHO was notified by the International Health Regulations (2005) National Focal Point for France through the European Commission (EC) Early Warning and Response System (EWRS) about a sharp increase in the number of dengue cases reported in Réunion, France since the beginning of 2018. Réunion is a French overseas territory in the Indian Ocean.
 NOAA: “Considered one of the world’s most active volcanoes, Piton de la Fournaise occupies the east-southeastern end of Réunion Island in the western Indian Ocean.”
NOAA: “Considered one of the world’s most active volcanoes, Piton de la Fournaise occupies the east-southeastern end of Réunion Island in the western Indian Ocean.”
As of 23 April, 1816 autochthonous dengue cases have been confirmed in Réunion in 2018, including 428 probable and confirmed cases reported from 16-23 April 2018; in comparison, less than 100 cases were reported in all of 2017. The western and southern parts of the island are the most affected. The number of emergency room visits and hospitalizations related to dengue fever have been increasing; 50 hospitalizations in 2018 have been reported compared to 12 hospitalizations for all of 2017. At this stage of the outbreak, the hospitals have the capacities to provide clinical care for all of the reported cases. Since the beginning of 2017, the main circulating strain is DENV-2 (537 serotypings); other serotypes have also been detected mainly among imported cases in 2017 (four DENV-1 and one DENV-4 serotypings). In 2014, DENV-2 was the main circulating serotype in 2014, along with DENV-1 and DENV-3; in 2016, DENV-1, was the main serotype along with DENV-2 and DENV-3.
Aedes albopictus and Aedes aegypti mosquitos are both found in Réunion; however, Ae. albopictus has historically had the highest observed abundance.
Public health response
Since 2006, notification of dengue infection to public authorities in Réunion is compulsory. Indian Ocean Regional Office of the French Institute for Public Health (Cire OI – Santé Publique France), in collaboration with the vector control team (Lutte anti-vectorielle, LAV) of the French Health Agency Indian Ocean (ARS-OI), is closely monitoring this outbreak. The following actions have been implemented in Réunion by public authorities:
- Reinforced vector control measures, focusing primarily in areas around reported dengue cases;
- Enhanced surveillance of cases;
- Blood and safety of substances of human origin (SoHo) safety measures implemented;
- Social mobilization activities; and
- Specific risk communication messages aiming to raise dengue fever awareness among the public and health care workers, including posters at points of entry (PoE) and announcements onboard flights to and from Réunion.
On 27 March 2018, public authorities raised the level of the emergency plan “Organisation de la Réponse de Sécurité Civile” (ORSEC) to 3, corresponding to a low-level epidemic. This plan includes:
- Active case finding;
- Intensified vector control;
- Reinforced communication to the public and health care workers;
- Mobilization of additional resources, including human resources (e.g., firefighters).
WHO risk assessment
Although sporadic autochthonous dengue fever cases and clusters have been reported in Réunion before, the upsurge of cases since the beginning of 2018 is unprecedented. This could be partly explained by:
- According to studies, in previous years asymptomatic cases contributed to the transmission cycle and since the proportion of asymptomatic cases was high, the virus has continued to spread unnoticed until now; and
- A lack of herd immunity in the local population, as only sporadic cases have been reported on the island before, thus favouring further transmission of the virus.
Réunion is a popular tourist destination and the likelihood of dengue virus introduction to other countries is heightened by the current outbreak.
The presence of competent mosquito vectors and the uncertainty regarding the level of dengue immunity of the local population highlight the need for enhanced risk communication and entomological and epidemiological surveillance to rapidly and effectively control the epidemic.
WHO advice
- WHO recommends enhancing Integrated Vector Management (IVM) activities to remove potential breeding sites, reduce vector populations and minimize individual exposures. This should include both vector control strategies (i.e. environmental management, and chemical and biological control measures), as well as strategies to protect individuals and households. For further information, see references below.
- Since the Aedes mosquitoes, are day-biting mosquitoes, personal protective measures such as the use of clothing that minimizes skin exposure during daylight hours is recommended. Repellents that contain DEET (diethyltoluamide), IR 3535 ((3- [N-butyl-N-acetyl], aminopropionic acid ethyl-ester) or KBR3023 (also called “Icaridin” or “Picaridin”) may be applied to exposed skin or to clothing. The use of repellents must be in strict accordance with label instructions.
- Insecticide-treated mosquito nets afford good protection for those who sleep during the day (e.g. infants, the bedridden and night-shift workers), but also during the night to prevent mosquito bites, if the lights are kept on.
- Where indoor biting occurs, household insecticide aerosol products, mosquito coils or other insecticide vaporizers may also reduce biting activity. Household fixtures such as windows, door screens and air-conditioning can also reduce biting.
- There is no specific treatment for dengue fever. Careful clinical detection and management of dengue patients can significantly reduce mortality rates from severe dengue.
- WHO advises against any restrictions on travel to or trade with Réunion based on the information available.
- Vector control strategies
- Global Strategy for dengue prevention and control, 2012–2020
- Dengue and severe dengue WHO fact sheet
- Santé Publique France Océan Indien (Cire OI)
U.S. trends in occurrence of nationally reportable vectorborne diseases during 2004–2016.
Wednesday, May 2nd, 2018Rosenberg R, Lindsey NP, Fischer M, et al. Vital Signs: Trends in Reported Vectorborne Disease Cases — United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep. ePub: 1 May 2018. DOI: http://dx.doi.org/10.15585/mmwr.mm6717e1.
Key Points
•A total of 642,602 cases of 16 diseases caused by bacteria, viruses, or parasites transmitted through the bites of mosquitoes, ticks, or fleas were reported to CDC during 2004–2016. Indications are that cases were substantially underreported.
•Tickborne diseases more than doubled in 13 years and were 77% of all vectorborne disease reports. Lyme disease accounted for 82% of all tickborne cases, but spotted fever rickettsioses, babesiosis, and anaplasmosis/ehrlichiosis cases also increased.
•Tickborne disease cases predominated in the eastern continental United States and areas along the Pacific coast. Mosquitoborne dengue, chikungunya, and Zika viruses were almost exclusively transmitted in Puerto Rico, American Samoa, and the U.S. Virgin Islands, where they were periodically epidemic. West Nile virus, also occasionally epidemic, was widely distributed in the continental United States, where it is the major mosquitoborne disease.
•During 2004–2016, nine vectorborne human diseases were reported for the first time from the United States and U.S. territories. The discovery or introduction of novel vectorborne agents will be a continuing threat.
•Vectorborne diseases have been difficult to prevent and control. A Food and Drug Administration–-approved vaccine is available only for yellow fever virus. Many of the vectorborne diseases, including Lyme disease and West Nile virus, have animal reservoirs. Insecticide resistance is widespread and increasing.
•Preventing and responding to vectorborne disease outbreaks are high priorities for CDC and will require additional capacity at state and local levels for tracking, diagnosing, and reporting cases; controlling vectors; and preventing transmission.
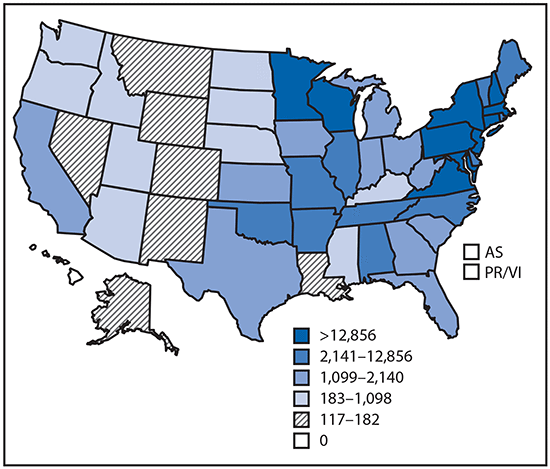
Reported cases* of tickborne disease — U.S. states and territories, 2004–2016
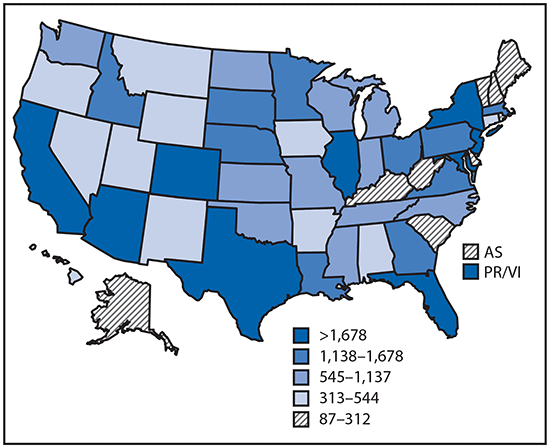
Reported cases* of mosquitoborne disease — U.S. states and territories, 2004–2016
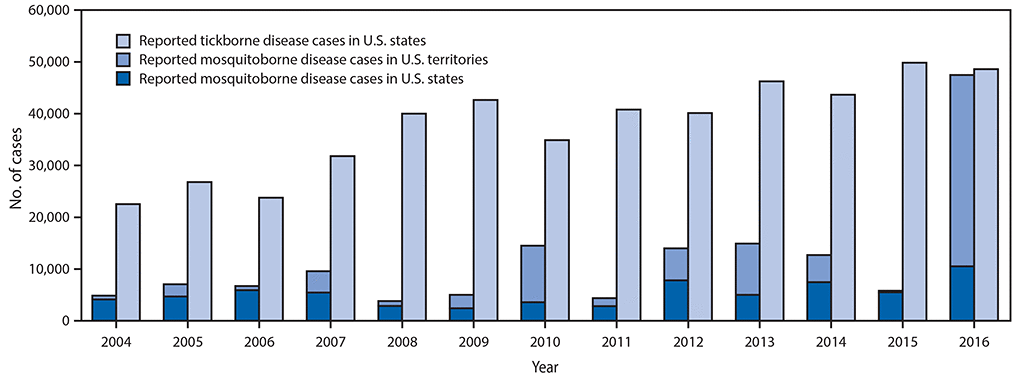
Reported nationally notifiable mosquitoborne,* tickborne, and fleaborne† disease cases — U.S. states and territories, 2004–2016
ADE (antibody dependent enhancement): The dengue phenomenon wherein antibodies from a previous dengue infection counterintuitively worsen subsequent infections with the flavivirus.
Friday, December 15th, 2017WHO advises Dengvaxia be used only in people previously infected with dengue

13 December 2017 – Following a consultation of the Global Advisory Committee on Vaccine Safety, the World Health Organization (WHO) finds that the dengue vaccine CYD-TDV, sold under the brand name Dengvaxia, prevents disease in the majority of vaccine recipients but it should not be administered to people who have not previously been infected with dengue virus.
This recommendation is based on new evidence communicated by the vaccine’s manufacturer (Sanofi Pasteur), indicating an increase in incidence of hospitalization and severe illness in vaccinated children never infected with dengue.
The WHO Global Advisory Committee on Vaccine Safety considered the company’s new results from clinical trial data analyses. Those studies indicate that increased risk of severe dengue disease in people who have never been infected affects about 15% of the vaccinated individuals. The magnitude of risk is in the order of about 4 out of every 1000 seronegative patients vaccinated who developed severe dengue disease during five years of observation. The risk of developing severe dengue disease in non-vaccinated individuals has been calculated as 1.7 per 1000 over the same period of observation. By contrast, for the 85% who have had dengue disease before immunization, there is a reduction of 4 cases of severe dengue per 1 000 who are vaccinated.
The possibility of risk for seronegative people was raised by WHO and published in a position paper in July 2016: “…vaccination may be ineffective or may theoretically even increase the future risk of hospitalized or severe dengue illness in those who are seronegative at the time of first vaccination regardless of age.”[i] As this risk had at that time not been seen in the age groups for which the vaccine was licensed, WHO issued a conditional recommendation, emphasizing the use of the vaccine in populations having been previously infected with dengue virus.
To minimize illness for seronegative vaccinated people, WHO recommends enhancing measures that reduce exposure to dengue infection among populations where the vaccine has already been administered. For vaccine recipients who present with clinical symptoms compatible with dengue virus infection, access to medical care should be expedited to allow for proper evaluation, identification, and management of severe forms of the disease.
Background
Dengue is a mosquito-borne flavivirus disease that has spread to most tropical and many subtropical areas. The disease is caused by four closely related viruses. There is no specific dengue treatment and prevention is mainly limited to vector control measures. A safe and effective dengue vaccine would therefore represent a major advance in the control of the disease.
CYD-TDV is a live attenuated tetravalent vaccine made using recombinant DNA technology and is administered in three phases separated by six-month intervals. It became commercially available in 2016 and is currently licensed in 19 countries.
[i] Dengue vaccine: WHO position paper – July 2016 http://www.who.int/wer/2016/wer9130.pdf
Dengue fever – Burkina Faso
Tuesday, November 7th, 2017
Dengue fever – Burkina Faso
Burkina Faso has been experiencing an epidemic of dengue fever since week 31 of 2017 (week ending on 6 August). WHO officially declared the outbreak on 28 September 2017. Unlike the previous occurrence in 2016, several serotypes of dengue have been detected and the number of cases quickly exceeded past seasonal outbreaks.
As of 27 October 2017, a total of 6 699 (suspected, probable or confirmed) cases and 13 deaths (CFR= 0.2%) were reported throughout the country. Of the total cases reported, 4 428 (66%) were probable, with a positive result for dengue by rapid diagnostic test (RDT), of 241 samples referred for PCR testing at the Viral Haemorrhagic fever (VHF) reference lab of the Centre Muraz, Bobo-Dioulasso, 141 (59%) were confirmed cases by positive result. Further characterization of 72 samples has identified three dengue virus serotypes: DENV-2 (N=58), DENV-3 (N=12) and DENV-1 (N=2). Cases are currently reported in 12 of the country’s 13 health regions, with 64% of cases reported in the central region, particularly in the city of Ouagadougou.
Public health response
- The National Epidemic Management Committee has been activated to coordinate response activities.
- Strengthening the epidemiological surveillance, and putting in place an Early Warning System that provides daily notification of cases in Ouagadougou and weekly in the other provinces.
- Provision of free medical care and treatment for severe cases in all hospitals (Regional and District hospitals). 5 000 RDTs distributed to reference health centers.
- Development and dissemination of a national dengue clinical management algorithm.
- Delivery and dissemination of key awareness and prevention measures through radio and television programs.
- Periodic shipment of samples to the VHF laboratory of the Centre Muraz, Bobo-Dioulasso.
- Implementation of intensive vector control interventions targeting larva and adult mosquitoes through.
- Elimination of mosquito breeding sites which was carried out by community volunteers in Ouagadougou;
- Distribution of 1 500 Long Lasting Insecticidal Nets (LLINs) to hospitals;
- Ongoing in-door spraying to control adult mosquitoes at the household level.
WHO risk assessment
This outbreak is occurring in the context of an improved but still limited dengue surveillance system in Burkina Faso. The weekly case incidence has been on the rise since the detection of the outbreak in week 31 and is likely underestimated. The epidemic has already spread to twelve of the country’s thirteen health zones and many public health facilities do not have access to dengue fever diagnostics (RDTs).
Burkina Faso experienced an outbreak of dengue in 2016 which was caused by DENV-2. In the current outbreak, three serotypes were identified: DENV-1, DENV-2, and DENV-3. This could lead to the occurrence of more severe cases, which may not be captured by the surveillance system due to under-reporting from private clinics and healthcare centers in peripheral districts.
The existence of multiple mosquito breeding sites after the rainy season may favor the continued presence of mosquito populations in the affected communities In particular, the peripheral districts of cities in Burkina Faso are characterized by poor sanitation and the accumulation of rubbish dump sites, tires and used containers which provide productive breeding sites for Aedes aegypti mosquitoes (the primary vector for dengue).
WHO advice
WHO recommends adequate and timely case management dengue cases. Surveillance should be strengthened within all affected health zones. Key public health communication messages should be provided. Integrated Vector Management (IVM) activities should be enhanced to remove potential breeding sites, reduce vector populations and minimize individual exposures. This should include both vector control strategies (i.e. environmental management, and chemical and biological control measures), as well as strategies to protect individuals and households. For further information, see:
WHO recommends that countries should consider the introduction of the dengue vaccine CYD-TDV only in geographic settings (national or subnational) where epidemiological data indicate a high burden of disease. Complete recommendations may be found in the WHO position paper on dengue vaccines:
WHO advises against any restriction on travel and trade to Burkina Faso or the affected district based on the available information.



