Archive for the ‘Malaria’ Category
WHO: 2018’s World Malaria Report at a Glance
Wednesday, November 21st, 2018This year’s World malaria report at a glance
The WHO’s 11th World malaria report summarizes global progress in the fight against malaria up to the end of 2017. The 2017 report showed that progress against malaria has stalled in many countries, and that the world was unlikely to achieve the WHO Global technical strategy for malaria 2016–2030 (GTS) morbidity and mortality targets for 2020. One year on, the 2018 report describes progress since then, including efforts to intensify the response in the highest burden countries.
Overview
Global and regional malaria burden, in numbers
Malaria cases
In 2017, an estimated 219 million cases of malaria occurred worldwide (95% confidence interval [CI]: 203–262 million), compared with 239 million cases in 2010 (95% CI: 219–285 million) and 217 million cases in 2016 (95% CI: 200–259 million).
Although there were an estimated 20 million fewer malaria cases in 2017 than in 2010, data for the period 2015–2017 highlight that no significant progress in reducing global malaria cases was made in this timeframe.
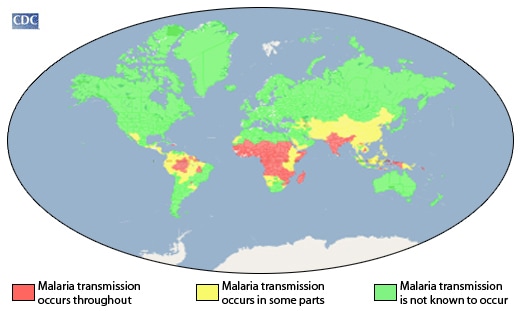
Most malaria cases in 2017 were in the WHO African Region (200 million or 92%), followed by the WHO South-East Asia Region with 5% of the cases and the WHO Eastern Mediterranean Region with 2%.
Fifteen countries in sub-Saharan Africa and India carried almost 80% of the global malaria burden. Five countries accounted for nearly half of all malaria cases worldwide: Nigeria (25%), Democratic Republic of the Congo (11%), Mozambique (5%), India (4%) and Uganda (4%).
The 10 highest burden countries in Africa reported increases in cases of malaria in 2017 compared with 2016. Of these, Nigeria, Madagascar and the Democratic Republic of the Congo had the highest estimated increases, all greater than half a million cases. In contrast, India reported 3 million fewer cases in the same period, a 24% decrease compared with 2016.
The incidence rate of malaria declined globally between 2010 and 2017, from 72 to 59 cases per 1000 population at risk. Although this represents an 18% reduction over the period, the number of cases per 1000 population at risk has stood at 59 for the past 3 years.
The WHO South-East Asia Region continued to see its incidence rate fall – from 17 cases of the disease per 1000 population at risk in 2010 to 7 in 2017 (a 59% decrease). All other WHO regions recorded either little progress or an increase in incidence rate. The WHO Region of the Americas recorded a rise, largely due to increases in malaria transmission in Brazil, Nicaragua and Venezuela (Bolivarian Republic of). In the WHO African Region, the malaria incidence rate remained at 219 cases per 1000 population at risk for the second year in a row.
Plasmodium falciparum is the most prevalent malaria parasite in the WHO African Region, accounting for 99.7% of estimated malaria cases in 2017, as well as in the WHO regions of South-East Asia (62.8%), the Eastern Mediterranean (69%) and the Western Pacific (71.9%). P. vivax is the predominant parasite in the WHO Region of the Americas, representing 74.1% of malaria cases.
Malaria deaths
In 2017, there were an estimated 435 000 deaths from malaria globally, compared with 451 000 estimated deaths in 2016, and 607 000 in 2010.
Children aged under 5 years are the most vulnerable group affected by malaria. In 2017, they accounted for 61% (266 000) of all malaria deaths worldwide.
The WHO African Region accounted for 93% of all malaria deaths in 2017. Although the WHO African Region was home to the highest number of malaria deaths in 2017, it also accounted for 88% of the 172 000 fewer global malaria deaths reported in 2017 compared with 2010.
Nearly 80% of global malaria deaths in 2017 were concentrated in 17 countries in the WHO African Region and India; 7 of these countries accounted for 53% of all global malaria deaths: Nigeria (19%), Democratic Republic of the Congo (11%), Burkina Faso (6%), United Republic of Tanzania (5%), Sierra Leone (4%), Niger (4%) and India (4%).
All WHO regions except the WHO Region of the Americas recorded reductions in mortality in 2017 compared with 2010. The largest declines occurred in the WHO regions of South- East Asia (54%), Africa (40%) and the Eastern Mediterranean (10%). Despite these gains, the malaria mortality reduction rate has also slowed since 2015, reflecting the estimated trends in malaria case incidence.
Malaria-related anaemia
This year’s report includes a section on malaria-related anaemia, a condition that, left untreated, can result in death, especially among vulnerable populations such as pregnant women and children aged under 5 years.
Anaemia was once a key indicator of progress in malaria control, and its prevalence was used to evaluate the efficacy of interventions. Recent years have seen a decline in awareness of the burden of malaria-associated anaemia
Despite its importance as a direct and indirect consequence of malaria, the prevalence of anaemia among populations vulnerable to the disease has not been reported consistently as a metric of malaria transmission and burden.
Data from household surveys conducted in 16 high-burden African countries between 2015 and 2017 show that, among children aged under 5 years, the prevalence of any anaemia was 61%, mild anaemia 25%, moderate anaemia 33% and severe anaemia 3%. Of children who tested positive for malaria, the prevalence of any anaemia was 79%, mild anaemia 21%, moderate anaemia 50% and severe anaemia 8%.
Investments in malaria programmes and research
Malaria control and elimination investments
In 2017, an estimated US$ 3.1 billion was invested in malaria control and elimination efforts globally by governments of malaria endemic countries and international partners – an amount slighter higher than the figure reported for 2016.
Nearly three quarters (US$ 2.2 billion) of investments in 2017 were spent in the WHO African Region, followed by the WHO regions of South-East Asia (US$ 300 million), the Americas (US$ 200 million), and the Eastern Mediterranean and the Western Pacific (US$ 100 million each).
In 2017, US$ 1.4 billion was invested in low-income countries, US$ 1.2 billion in lower-middle income countries and US$ 300 million in upper-middle-income countries. International funding represented the major source of funding in low-income and lower-middle-income countries, at 87% and 70%, respectively.
Governments of endemic countries contributed 28% of total funding (US$ 900 million) in 2017, a figure unchanged from 2016. Two thirds of domestically sourced funds were invested in malaria control activities carried out by national malaria programmes (NMPs), with the remaining share estimated as the cost of patient care.
As in previous years, the United States of America (USA) was the largest international source of malaria financing, providing US$ 1.2 billion (39%) in 2017. Country members of the Development Assistance Committee together accounted for US$ 700 million (21%). The United Kingdom of Great Britain and Northern Ireland contributed around US$ 300 million (9%) while the Bill & Melinda Gates Foundation provided US$ 100 million (2%).
Of the US$ 3.1 billion invested in 2017, US$ 1.3 billion was channelled through the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Investment outlook
Although funding for malaria has remained relatively stable since 2010, the level of investment in 2017 is far from what is required to reach the first 2 milestones of the GTS; that is, a reduction of at least 40% in malaria case incidence and mortality rates globally by 2020, compared with 2015 levels.
To reach the GTS 2030 targets, it is estimated that annual malaria funding will need to increase to at least US$ 6.6 billion per year by 2020.
Stepping up investments in malaria research and development is key to achieving the GTS targets. In 2016, US$ 588 million was spent in this area, representing 85% of the estimated annual need for research and development.
Although research and development funding for malaria vaccines and drugs declined in 2016 compared with 2015, investments in vector control products almost doubled, from US$ 33 million to US$ 61 million.
Deliveries of malaria commodities
Insecticide-treated mosquito nets
Between 2015 and 2017, a total of 624 million insecticide-treated mosquito nets (ITNs), mainly long-lasting insecticidal nets (LLINs), were reported by manufacturers as having been delivered globally. This represents a substantial increase over the previous period 2012–2014, when 465 million ITNs were delivered globally.
An estimated 552 million ITNs were distributed by NMPs globally, with most (459 million or 83%) being delivered in sub-Saharan Africa over the period 2015–2017.
Globally, 85% of ITNs were distributed through free mass distribution campaigns, 8% in antenatal care facilities and 4% as part of immunization programmes.
Rapid diagnostic tests
An estimated 276 million rapid diagnostic tests (RDTs) were sold globally in 2017.
In 2017, 245 million RDTs were distributed by NMPs. Most RDTs (66%) were tests that detected P. falciparum only and were supplied to sub-Saharan Africa.
In sub-Saharan Africa, RDTs are becoming increasingly the most used method to test for malaria diagnosis among suspected malaria patients in public health facilities. In 2017, an estimated 75% of malaria tests were conducted using RDTs, up from 40% in 2010.
Artemisinin-based combination therapy
An estimated 2.74 billion treatment courses of artemisinin-based combination therapy (ACT) were procured by countries over the period 2010–2017. An estimated 62% of these procurements were reported to have been made for the public sector.
During the period 2010–2017, 1.45 billion ACT treatment courses were delivered by NMPs, of which 1.42 billion (98%) were in the WHO African Region.
With increases in diagnostic testing in recent years, ACT treatment courses are becoming more targeted towards patients who tested positive for malaria. This is demonstrated by a substantially reduced ratio of ACTs to tests (0.8 in 2017 compared with 2.5 in 2010). Nevertheless, this implies that an estimated 30% of patients who received ACTs were not tested for malaria.
Preventing malaria
Vector control
Half of people at risk of malaria in Africa are sleeping under an ITN: in 2017, 50% of the population was protected by this intervention, an increase from 29% in 2010. Furthermore, the percentage of the population with access to an ITN increased from 33% in 2010 to 56% in 2017. However, coverage has improved only marginally since 2015 and has been at a standstill since 2016.
Households with at least 1 ITN for every 2 people doubled to 40% between 2010 and 2017. However, this figure represents only a modest increase over the past 3 years, and remains far from the target of universal coverage.
Fewer people at risk of malaria are being protected by indoor residual spraying (IRS), a prevention method that involves spraying the inside walls of dwellings with insecticides. Globally, IRS protection declined from a peak of 5% in 2010 to 3% in 2017, with decreases seen across all WHO regions.
In the WHO African Region, IRS coverage dropped from 80 million people at risk in 2010, to a low point of 51 million in 2016 before rising to 64 million in 2017. In other WHO regions, the number of people protected with IRS in 2017 was 1.5 million in the Americas, 7.5 million in the Eastern Mediterranean, 41 million in South-East Asia, and 1.5 million in the Western Pacific.
The declines in IRS coverage are occurring as countries change or rotate insecticides (changing to more expensive chemicals), and as operational strategies change (e.g. decreasing at-risk populations in malaria elimination countries).
Preventive therapies
To protect women in areas of moderate and high malaria transmission in Africa, WHO recommends “intermittent preventive treatment in pregnancy” (IPTp) with the antimalarial drug sulfadoxine-pyrimethamine. Among 33 African countries that reported on IPTp coverage levels in 2017, an estimated 22% of eligible pregnant women received the recommended 3 or more doses of IPTp, compared with 17% in 2015 and 0% in 2010.
In 2017, 15.7 million children in 12 countries in Africa’s Sahel subregion were protected through seasonal malaria chemoprevention (SMC) programmes. However, about 13.6 million children who could have benefited from this intervention were not covered, mainly due to a lack of funding.
Diagnostic testing and treatment
Accessing care
Prompt diagnosis and treatment is the most effective way to prevent a mild case of malaria from developing into severe disease and death. Based on national household surveys completed in 19 countries in sub-Saharan Africa between 2015 and 2017, a median of 52% (interquartile range [IQR]: 44–62%) of children with a fever (febrile) were taken to a trained medical provider for care. This includes public sector hospitals and clinics, formal private sector health facilities and community health workers.
Although more febrile children were brought for care in the public health sector (median: 36%, IQR: 30–46%) than in the formal medical private sector (median: 8%, IQR: 5–10%), a high proportion of febrile children did not receive any medical attention (median: 40%, IQR: 28–45%). Poor access to health care providers or lack of awareness of malaria symptoms among caregivers are among the contributing factors.
The national surveys reveal disparities in access to health care based on household income and location: the percentage of febrile children brought for care was higher in wealthier households (median: 72%, IQR: 62–75%) compared with poorer households (median: 58%, IQR: 47–67%), and was higher among those living in urban areas (median: 69%, IQR: 59–76%) compared with rural areas (median: 60%, IQR: 51–71%).
Diagnosing malaria
According to 58 surveys conducted in 30 sub-Saharan African countries between 2010 and 2017, the percentage of children with a fever that received a diagnostic test in the public health sector has increased, hitting a median of 59% (IQR: 34–75%) over the period 2015– 2017, up from a median of 33% (IQR:18–44%) for 2010–2012.
Data collected from 56 surveys carried out in sub-Saharan Africa reveal that the percentage of febrile children attending public health facilities who received a malaria diagnostic test before antimalarial treatment has gone up from a median of 35% (IQR: 27–56%) in 2010–2012 to 74% (IQR: 51–81%) in 2015–2017. A similar increase has been recorded in the formal private health sector, from 41% (IQR: 17–67%) in 2010–2012 to 63% (IQR: 41–83%) in 2015–2017.
Treating malaria
Based on 19 household surveys conducted in sub-Saharan Africa between 2015 and 2017, the percentage of children aged under 5 years with a fever who received any antimalarial drug was 29% (IQR: 15–48%).
Children are more likely to be given ACTs – the most effective antimalarial drugs – if medical care is sought in the public sector compared with the private sector. Data from 18 national surveys conducted in sub-Saharan Africa show that for the period 2015–2017, an estimated 88% (IQR: 73–92%) of febrile children brought for treatment for malaria in the public health sector received ACTs, compared with 74% (IQR: 47–88%) in the formal medical private sector.
To bridge the treatment gap among children, WHO recommends the uptake of integrated community case management (iCCM). This approach promotes integrated management of common life-threatening conditions in children – malaria, pneumonia and diarrhoea – at health facility and community levels. In 2017, of 21 African countries with high malaria burden, 20 had iCCM policies in place, of which 12 had started implementing those policies.
Malaria surveillance systems
Effective surveillance of malaria cases and deaths is essential for identifying the areas or population groups that are most affected by malaria, and for targeting resources for maximum impact. A strong surveillance system requires high levels of access to care and case detection, and complete reporting of health information by all sectors, whether public or private.
In 2017, among 52 moderate to high-burden countries, reporting rates of malaria were 60% or more. In the WHO African Region, 36 out of 46 countries indicated that at least 80% of public health facilities had reported data on malaria through their national health information system.
Malaria elimination
Globally, the elimination net is widening, with more countries moving towards zero indigenous cases: in 2017, 46 countries reported fewer than 10 000 such cases, up from 44 countries in 2016 and 37 countries in 2010. The number of countries with less than 100 indigenous cases – a strong indicator that elimination is within reach – increased from 15 countries in 2010 to 24 countries in 2016 and 26 countries in 2017.
Paraguay was certified by WHO as malaria free in 2018, while Algeria, Argentina and Uzbekistan have made formal requests to WHO for certification. In 2017, China and El Salvador reported zero indigenous cases.
One of the key GTS milestones for 2020 is elimination of malaria in at least 10 countries that were malaria endemic in 2015. At the current rate of progress, it is likely that this milestone will be reached.
In 2016, WHO identified 21 countries with the potential to eliminate malaria by the year 2020. WHO is working with the governments in these countries – known as “E-2020 countries” – to support their elimination acceleration goals.
Although 11 E-2020 countries remain on track to achieve their elimination goals, 10 have reported increases in indigenous malaria cases in 2017 compared with 2016.
Challenges in getting the malaria response back on track
The challenges facing the global malaria response are many, and as highlighted in this year’s report, immediate barriers to achieving the fast-approaching GTS milestones for 2020 and 2025 are malaria’s continued rise in countries with the highest burden of the disease and inadequate international and domestic funding. At the same time, the continued emergence of parasite resistance to antimalarial medicines and mosquito resistance to insecticides pose threats to progress.
High-burden countries
In 2017, 11 countries accounted for approximately 70% of estimated malaria cases and deaths globally: 10 in sub-Saharan Africa and India. Among these countries, only India reported progress in reducing its malaria cases in 2017 compared to 2016.
To get the global malaria response back on track, a new country-driven approach – “High burden to high impact” – will be launched in Mozambique on 19 November 2018, alongside the release of the World malaria report 2018.
Catalyzed by WHO and the RBM Partnership to End Malaria, the approach is founded upon 4 pillars: galvanize national and global political attention to reduce malaria deaths; drive impact in country through the strategic use of information; establish best global guidance, policies and strategies suitable for all malaria endemic countries; and implement a coordinated country response.
Funding
In 24 out of 41 high-burden countries, which rely mainly on external funding for malaria programmes, the average level of funding available per person at risk declined in 2015–2017 compared to 2012–2014. This ranged from a 95% reduction in the Congo (highest) to a 1% decrease in Uganda (lowest) over the time points compared.
In the countries that experienced a 20% or more decrease in total funding per person at risk, international financing declined, at times combined with lower domestic investments.
Among the 41 high-burden countries, overall, funding per person at risk of malaria stood at US$ 2.32.
Drug resistance
ACTs have been integral to the recent success of global malaria control, and protecting their efficacy for the treatment of malaria is a global health priority.
Most studies conducted between 2010 and 2017 show that ACTs remain effective, with overall efficacy rates greater than 95% outside the Greater Mekong subregion (GMS). In Africa, artemisinin (partial) resistance has not been reported to date.
Although multidrug resistance, including artemisinin (partial) resistance and partner drug resistance, has been reported in 4 GMS countries, there has been a massive reduction in malaria cases and deaths in this subregion. Monitoring the efficacy of antimalarial drugs has resulted in prompt updating of malaria treatment policies in most GMS countries.
Insecticide resistance
The recently released WHO Global report on insecticide resistance in malaria vectors: 2010– 2016 showed that resistance to the four commonly used insecticide classes – pyrethroids, organochlorines, carbamates and organophosphates – is widespread in all major malaria vectors across the WHO regions of Africa, the Americas, South-East Asia, the Eastern Mediterranean and the Western Pacific.
Of the 80 malaria endemic countries that provided data for 2010–2017, resistance to at least 1 of the 4 insecticide classes in 1 malaria vector from 1 collection site was detected in 68 countries, an increase over 2016 due to improved reporting and 3 new countries reporting on resistance for the first time. In 57 countries, resistance to 2 or more insecticide classes was reported.
Resistance to pyrethroids – the only insecticide class currently used in ITNs – is widespread and was detected in at least 1 malaria vector in more than two thirds of the sites tested and was highest in the WHO regions of Africa and the Eastern Mediterranean.
Resistance to organochlorines was detected for at least 1 malaria vector in almost two thirds of the sites and was highest in the WHO South-East Asia Region. Resistance to carbamates and organophosphates was less prevalent and was detected in 33% and 27% of the tested sites, respectively. Prevalence was highest for carbamates in the WHO South-East Asia Region and for organophosphates in the WHO Western Pacific Region.
In view of the current situation, resistance monitoring and management plans are essential, in line with the WHO Global plan for insecticide resistance management in malaria vectors. To date, 40 countries have completed these plans.
ITNs continue to be an effective tool for malaria prevention, even in areas where mosquitoes have developed resistance to pyrethroids. This was evidenced in a large multicountry evaluation coordinated by WHO between 2011 and 2016 across study locations in 5 countries.
Tedros Adhanom Ghebreyesus, PhD, the WHO’s director-general: “Nobody should die from malaria.”
Tuesday, November 20th, 2018- Last year, about 70% of malaria cases and deaths were concentrated in 11 countries: Ten are in Africa (Burkina Faso, Cameroon, Democratic Republic of the Congo, Ghana, Mali, Mozambique, Niger, Nigeria, Uganda, and Tanzania), and the other is India.
- The 10 African nations had 3.5 million more malaria infections than in 2016
- India showed progress in reducing its disease burden.
- Possible reasons for the increase in vulnerable countries: Major coverage gaps in use of insecticide-treated bed nets and other tools for preventing the mosquito-borne disease.
- WHO estimated that for 2017, half of Africa’s at-risk populations did not sleep under a treated bed net. Fewer homes in the region were protected by indoor residual spraying and that use of therapies for protecting pregnant women and children from malaria was still too low.
- Funding for the global response has leveled off. For 2017, there was $3.1 billion for malaria control and elimination programs, 28% of it from the governments of malaria-endemic countries.
- The United States was still the single largest international donor, contributing $1.2 billion (39%) toward malaria efforts in 2017.
- At least $6.6 billion annually by 2020 is needed, which the WHO said is more than double the amount currently available.
Glimmers of progress elsewhere
- More countries are nearing malaria elimination. There were 46 in 2017, compared with 37 in 2010.
- China and El Salvador, two malaria-endemic countries, reported no local transmission in 2017
- This year, the WHO certified Paraguay as malaria-free, the first Americas country to achieve the status in 45 years.
- The WHO said three other countries have requested WHO malaria-free certification: Algeria, Argentina, and Uzbekistan.
- India reported a 24% reduction in cases for 2017 compared with the previous year.
- Other nations reporting declines in cases last year included Rwanda, Ethiopia, and Pakistan.
Is this the end of malaria???????
Tuesday, September 25th, 2018“…..In 2016 the disease, which is caused by a parasite and transmitted by mosquitoes, infected 194 million people in Africa and caused 445,000 deaths.
But biologists now have developed a way of manipulating mosquito genetics that forces whole populations of the insect to self-destruct. The technique has proved so successful in laboratory tests that its authors envisage malaria could be eliminated from large regions of Africa within two decades…..”
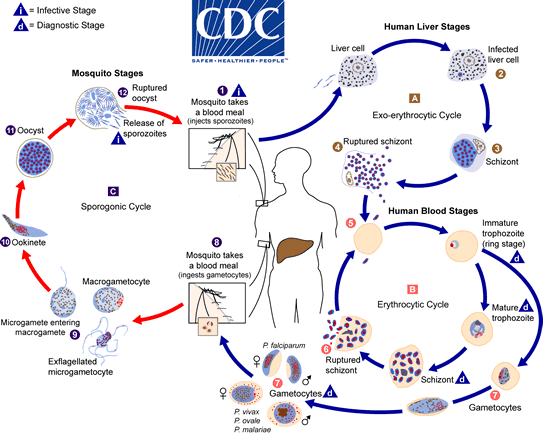
The malaria parasite life cycle involves two hosts. During a blood meal, a malaria-infected female Anopheles mosquito inoculates sporozoites into the human host  . Sporozoites infect liver cells
. Sporozoites infect liver cells  and mature into schizonts, which rupture and release merozoites
and mature into schizonts, which rupture and release merozoites  . (Of note, in P. vivax and P. ovale a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later.) After this initial replication in the liver (exo-erythrocytic schizogony
. (Of note, in P. vivax and P. ovale a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later.) After this initial replication in the liver (exo-erythrocytic schizogony  ), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony
), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony  ). Merozoites infect red blood cells
). Merozoites infect red blood cells  . The ring stage trophozoites mature into schizonts, which rupture releasing merozoites
. The ring stage trophozoites mature into schizonts, which rupture releasing merozoites  . Some parasites differentiate into sexual erythrocytic stages (gametocytes)
. Some parasites differentiate into sexual erythrocytic stages (gametocytes)  . Blood stage parasites are responsible for the clinical manifestations of the disease. The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal
. Blood stage parasites are responsible for the clinical manifestations of the disease. The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal  . The parasites’ multiplication in the mosquito is known as the sporogonic cycle
. The parasites’ multiplication in the mosquito is known as the sporogonic cycle  . While in the mosquito’s stomach, the microgametes penetrate the macrogametes generating zygotes
. While in the mosquito’s stomach, the microgametes penetrate the macrogametes generating zygotes  . The zygotes in turn become motile and elongated (ookinetes)
. The zygotes in turn become motile and elongated (ookinetes)  which invade the midgut wall of the mosquito where they develop into oocysts
which invade the midgut wall of the mosquito where they develop into oocysts  . The oocysts grow, rupture, and release sporozoites
. The oocysts grow, rupture, and release sporozoites , which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites
, which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites  into a new human host perpetuates the malaria life cycle.
into a new human host perpetuates the malaria life cycle.
Oxitec, Ltd. (“Oxitec”) is entering into a cooperative agreement with the Bill & Melinda Gates Foundation to develop a new strain of Oxitec’s self-limiting Friendly™ Mosquitoes to combat a mosquito species that spreads malaria
Thursday, June 21st, 2018“…….Oxitec will be using its new 2nd generation Friendly™ Mosquito technology to develop an Anopheles albimanus strain to address one of the most important vectors of malaria in the Americas. All of Oxitec’s Friendly™ Mosquito strains are designed to significantly reduce the population of a targeted mosquito species in the wild without impact on human or environmental health. Upon release into the wild, Oxitec’s 2nd generation male-selecting strains mate with wild females, and only male offspring with a self-limiting gene survive to adulthood. The female offspring from these matings – only female mosquitoes bite – will die before reaching adulthood. The surviving non-biting males subsequently seek out and mate with more wild females and pass along the self-limiting trait for up to ten generations before no longer persisting in the environment. When deployed as part of an integrated vector control program, this strain is anticipated to dramatically reduce wild populations of this malaria-transmitting mosquito species, while still ensuring Oxitec self-limiting mosquitoes do not persist in the environment...…..”
New anti-malarial netting: A long-lasting insecticidal net that incorporates a synergist piperonyl butoxide (PBO) and a long-lasting indoor residual spraying formulation of the insecticide pirimiphos-methyl.
Friday, May 11th, 2018“……The PBO long-lasting insecticidal net and non-pyrethroid indoor residual spraying interventions showed improved control of malaria transmission compared with standard long-lasting insecticidal nets where pyrethroid resistance is prevalent……”

“….The new nets contain pyrethroids, a class of chemicals used in nets for over a decade, along with the newer compound, piperonyl butoxide, which blocks mosquitoes’ ability to break down pyrethroids.….”

Between January 2016 and April 2018, six sporadic malaria hospital transmissions have been identified in the EU, in four Member States: Italy (2 cases), Spain (2 cases), Greece (1 case) and Germany (1 case).
Tuesday, May 1st, 2018“…..According to the scientific literature, the following modes of transmission should be taken into account in the investigation of hospital-acquired malaria:
- Parenteral introduction of blood that contains parasite-infected erythrocytes from one infectious individual to another patient during healthcare procedures;
- Blood transfusion, or bone marrow or organ transplant from a malaria-infected patient;
- Accidental contact of blood containing parasite-infected erythrocytes with an open wound…..”
Methylene blue & Malaria
Sunday, February 11th, 2018“……Methylene blue, a dye used to stain tissues viewed under a microscope, can be taken by tablet or injection, and is sometimes used to treat urethral infections and a hemoglobin disorder.

But the dye also kills the malaria parasites in the gametocyte stage, the point at which mosquitoes pick it up from human blood and pass it on to new victims.
Most malaria drugs do not target gametocytes, meaning that someone may still spread the disease for a week or more after treatment……”
Increase of malaria in the Americas
Thursday, February 1st, 2018Following a continued decrease in the number of malaria cases from 2005 to 2014 in the Region of the Americas, an increase was observed in 2015, 2016, and most recently in 2017. In 2016, 9 countries of the Region (Colombia, Ecuador, El Salvador, Guyana, Haiti, Honduras, Nicaragua, Panama, and the Bolivarian Republic of Venezuela) reported an increase in malaria cases.
In 2017, five countries reported an increase in malaria cases: Brazil, Ecuador, Mexico, Nicaragua, and Venezuela. In addition, Cuba and Costa Rica reported indigenous cases and Honduras reported malaria cases in an area where cases had not been detected recently. Following are summaries of the malaria situation in several countries of the Region.
In Brazil, the International Health Regulations (IHR) National Focal Point reported that between January and November of 2017, there were 174,522 malaria cases reported in the Amazon region, representing an increase in comparison to the same period of 2016 when 117,832 malaria cases were reported. In 2017, the same states, with the exception of Mato Grosso, presented an increase compared to 2016 (Table 1). The states reporting the most cases were Amazonas, Pará, and Acre. In 2017, 10% (17,411 cases) of the reported malaria cases in the Amazon region, correspond to malaria due to P. falciparum and mixed infections, representing a total higher than that reported for the same period in 2015 (14,084) and in 2016 (12,366).
In Costa Rica, the Ministry of Health reported 12 indigenous cases of malaria in 2017, in the cantons of San Carlos (6 cases), Matina (3 cases), and Sarapiqui (3 cases). This represents an increase compared to 2016 when 4 indigenous cases were notified.1,2 The detection of cases in these localities highlights the risk of re-establishment of transmission in areas where ecological conditions persist.
In Ecuador, between epidemiological week (EW) 1 and EW 52 of 2017, a total of 1,279 malaria cases were reported, of these 72% correspond to P. vivax and 28% to P. falciparum.3 The number of cases reported in 2017 is higher than that reported in 2016 (926).4 The four provinces with the highest number of cases in 2017 were Morona Santiago (489), followed by Orellana (240), Pastaza (223), and Esmeraldas (215).
In Honduras, the IHR National Focal Point reported the first indigenous cases of P. vivax malaria on 30 August 2017 in the village of La Charamusca, municipality of Esquías, department of Comayagua. A total of 34 confirmed cases were reported with date of onset of symptoms between EW 27 and 37 of 2017. During the outbreak investigation, the presence of Anopheles pseudopunctipennis was reported as a vector that could be involved in the transmission. The low number of cases registered in the department of Comayagua in the last five years and the absence of transmission for several years in the affected locality, highlights the importance of maintaining surveillance and response capabilities in areas where transmission has been interrupted.
In Mexico, the Secretariat of Health notified 704 malaria cases between EW 1 to EW 50 of 2017, representing an increase from the 514 cases reported in the same period of 2016.5 The increase was particularly notable in the states of Chiapas, Chihuahua, and Tabasco, and highlighted are cases in territories without recent transmission (San Luis Potosí).
In Nicaragua, between EW 1 and EW 52 of 2017, there were 10,846 malaria cases reported, representing an increase compared to the same period in 2016 when 6,209 cases were reported.6 The majority of the cases have been reported from the North Caribbean Coast Autonomous Region.7
The Venezuela, IHR National Focal Point notified the Pan American Health Organization, Regional Office of the World Health Organization (PAHO/WHO), on 27 November 2017, that between EW 1 and EW 42 of 2017 there were 319,765 malaria cases reported between EW and EW 42 of 2017 (Figure 1); representing an increase in comparison to the accumulated reported cases in 2016 (240,613).8
Of the cases reported in 2017, 77% were due to P. vivax, 17% due to P. falciparum, 6% due to mixed infections, and <1% due to P. malariae.
The number of malaria cases reported in 2017 was higher than the annual average recorded in the past 29 years (1988-2016).9
The three states with the highest number of confirmed cases during 2017 were Bolívar (205,215), followed by Amazonas (52,471) and Sucre (45,622). The majority of municipalities in these three states are characterized as having very high and high risk of malaria transmission according to the annual parasitic incidence (API). The risk of malaria is highest in those of 20 to 39 years and account for nearly half of all the cases (47%), showing the risk related to economic activities. Of the total confirmed cases, 64% (203,956) are male and across all age groups more cases in males were reported than in females
Yemen: The World Health Organization estimated that malaria cases rose in 2016 to 433,000 from 336,000 the year before.
Saturday, January 20th, 2018

The malaria parasite life cycle involves two hosts. During a blood meal, a malaria-infected female Anopheles mosquito inoculates sporozoites into the human host  . Sporozoites infect liver cells
. Sporozoites infect liver cells  and mature into schizonts
and mature into schizonts  , which rupture and release merozoites
, which rupture and release merozoites  . (Of note, in P. vivax and P. ovale a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later.) After this initial replication in the liver (exo-erythrocytic schizogony
. (Of note, in P. vivax and P. ovale a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later.) After this initial replication in the liver (exo-erythrocytic schizogony 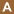 ), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony
), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony  ). Merozoites infect red blood cells
). Merozoites infect red blood cells  . The ring stage trophozoites mature into schizonts, which rupture releasing merozoites . Some parasites differentiate into sexual erythrocytic stages (gametocytes)
. The ring stage trophozoites mature into schizonts, which rupture releasing merozoites . Some parasites differentiate into sexual erythrocytic stages (gametocytes)  . Blood stage parasites are responsible for the clinical manifestations of the disease.
. Blood stage parasites are responsible for the clinical manifestations of the disease.
The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal  . The parasites’ multiplication in the mosquito is known as the sporogonic cycle
. The parasites’ multiplication in the mosquito is known as the sporogonic cycle  . While in the mosquito’s stomach, the microgametes penetrate the macrogametes generating zygotes
. While in the mosquito’s stomach, the microgametes penetrate the macrogametes generating zygotes  . The zygotes in turn become motile and elongated (ookinetes)
. The zygotes in turn become motile and elongated (ookinetes)  which invade the midgut wall of the mosquito where they develop into oocysts
which invade the midgut wall of the mosquito where they develop into oocysts  . The oocysts grow, rupture, and release sporozoites
. The oocysts grow, rupture, and release sporozoites  , which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites
, which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites  into a new human host perpetuates the malaria life cycle.
into a new human host perpetuates the malaria life cycle.
Treatment of Malaria: Guidelines For Clinicians (United States)
Friday, December 29th, 2017- Download PDF version of Parts 1-3 formatted for print[PDF, 79 KB, 8 pages]
- Part 1: Reporting and Evaluation & Diagnosis
- Part 2: Treatment: General Approach to Treatment and Treatment of Uncomplicated Malaria
- Part 3: Alternatives for Pregnant Women and Treatment of Severe Malaria
- Treatment algorithm
Treatment summary in decision tree form[PDF, 38 KB, 1 page] - Treatment Guidelines Table
Treatment summary in tabular form (Updated May, 2009) Download PDF version formatted for print[PDF, 154 KB, 3 pages]
Malaria can be a severe, potentially fatal disease (especially when caused by Plasmodium falciparum) and treatment should be initiated as soon as possible.
Patients who have severe P. falciparum malaria or who cannot take oral medications should be given the treatment by continuous intravenous infusion.
Most drugs used in treatment are active against the parasite forms in the blood (the form that causes disease) and include:
- chloroquine
- atovaquone-proguanil (Malarone®)
- artemether-lumefantrine (Coartem®)
- mefloquine (Lariam®)
- quinine
- quinidine
- doxycycline (used in combination with quinine)
- clindamycin (used in combination with quinine)
- artesunate (not licensed for use in the United States, but available through the CDC malaria hotline)
In addition, primaquine is active against the dormant parasite liver forms (hypnozoites) and prevents relapses. Primaquine should not be taken by pregnant women or by people who are deficient in G6PD (glucose-6-phosphate dehydrogenase). Patients should not take primaquine until a screening test has excluded G6PD deficiency.
How to treat a patient with malaria depends on:
- The type (species) of the infecting parasite
- The area where the infection was acquired and its drug-resistance status
- The clinical status of the patient
- Any accompanying illness or condition
- Pregnancy
- Drug allergies, or other medications taken by the patient
Report a serious drug side effect
If you have had a serious side effect while taking a drug, you or your health care provider can report that side effect to the federal Food and Drug Administration (FDA). MedWatch is the FDA Safety Information and Adverse Event Reporting Program. You are encouraged to take the reporting form to your health care provider.
Alternatively, health care providers can report to the FDA.
- online www.accessdata.fda.gov/scripts/medwatch/
- by phone (1-800-FDA-1088)
- or fax (1-800-FDA-0178)
The advantage to having your health care provider file the report is that he/she can provide clinical information based on your medical record that can help the FDA evaluate the report.
However, for a variety of reasons, you may not wish to have the form completed by your provider, or the provider may not wish to complete the form. Your health care provider is not required to report to the FDA. In this case, you may complete the online reporting form at www.fda.gov/medwatch/report/consumer/consumer.htm yourself via the Internet.
Related Links
The CDC malaria diagnosis and treatment guidelines have also been published in an article in JAMA May 23, 2007 and can be accessed for free online: view JAMA article.

