Archive for June, 2017
Globally, deaths due to diarrhoeal diseases have decreased substantially in the past 25 years
Saturday, June 3rd, 2017Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015
Evidence before this study
This manuscript builds on previous GBD publications with updated data and methods. Diarrhoeal diseases are a leading cause of morbidity and mortality, especially in children younger than 5 years, and the global burden has been estimated by several groups, including the Maternal and Child Health Epidemiology Estimation group and the Global Burden of Disease Study (GBD) 2013. Diarrhoea mortality has declined substantially since 1990, but morbidity has not declined as rapidly. Diarrhoeal mortality attributable to aetiologies has mainly been based on categorical attribution from non-molecular diagnostic methods with low overall attribution.
Added value of this study
This study provides a comprehensive assessment of diarrhoea burden based on the findings of GBD 2015, including new and more robust evidence on the mortality, morbidity, and risk factors associated with diarrhoea and 13 aetiologies and is the first cause-specific description of diarrhoea from the GBD group. Moreover, it introduces molecular diagnostic case definitions for diarrhoeal aetiologies. In addition to descriptions of trends in morbidity and mortality, this analysis uses a Socio-demographic Index to relate changes in diarrhoea burden to demographic transitions and assesses the effect of changing population characteristics and risk factor exposure to decompose trends in diarrhoea mortality.
Implications of all the available evidence
This study provides a detailed picture of the decreasing diarrhoeal burden over time and 13 aetiologies across all geographies while relating these trends to changes in risk factor exposure. This work allows for an in-depth understanding of national health challenges and areas for intervention. The findings will have great implications for strategies and programmes to address the burden of diarrhoea at the global, country, and local level.
USA: High-level isolation units in select Ebola hospitals report struggling to fund ongoing operations and sustain readiness.
Saturday, June 3rd, 2017Volume 23, Number 6—June 2017
Dispatch
Sustainability of High-Level Isolation Capabilities among US Ebola Treatment Centers
Main Article(https://wwwnc.cdc.gov/eid/article/23/6/17-0062_article)
Figure 1
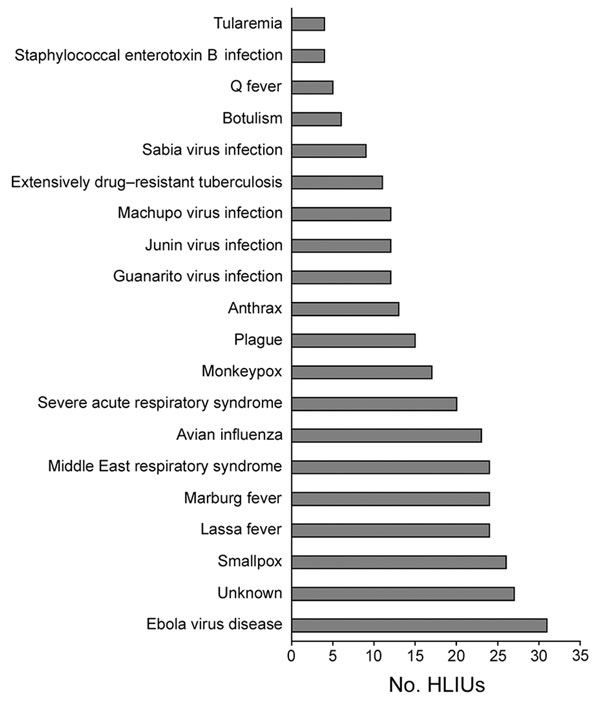
Figure 1. Diseases that 31 HLIUs reported they would treat, United States, 2016. HLIU, high-level isolation unit.
Main Article(https://wwwnc.cdc.gov/eid/article/23/6/17-0062_article)
South Sudan: 15 children die in botched measles vaccine campaign
Friday, June 2nd, 2017- The health ministry blamed the deaths on human error.
- One syringe was used for all the children
- The vaccine was not stored properly.
- All of the children who died were under the age of 5.
A gunman stormed a crowded Manila casino early Friday and used gasoline to set gambling tables on fire, creating clouds of smoke that swept through the crowds and killed at least 36
Friday, June 2nd, 2017WHO: New vector control response seen as game-changer
Friday, June 2nd, 2017The call came from the WHO Director-General in May 2016 for a renewed attack on the global spread of vector-borne diseases.
“What we are seeing now looks more and more like a dramatic resurgence of the threat from emerging and re-emerging infectious diseases,” Dr Margaret Chan told Member States at the Sixty-ninth World Health Assembly. “The world is not prepared to cope.”
Dr Chan noted that the spread of Zika virus disease, the resurgence of dengue, and the emerging threat from chikungunya were the result of weak mosquito control policies from the 1970s. It was during that decade that funding and efforts for vector control were greatly reduced.
‘Vector control has not been a priority’
Dr Ana Carolina Silva Santelli has witnessed this first-hand. As former head of the programme for malaria, dengue, Zika and chikungunya with Brazil’s Ministry of Health, she saw vector-control efforts wane over her 13 years there. Equipment such as spraying machines, supplies such as insecticides and personnel such as entomologists were not replaced as needed. “Vector control has not been a priority,” she said.
Today more than 80% of the world’s population is at risk of vector-borne disease, with half at risk of two or more diseases. Mosquitoes can transmit, among other diseases, malaria, lymphatic filariasis, Japanese encephalitis and West Nile; flies can transmit onchocerciasis, leishmaniasis and human African trypanosomiasis (sleeping sickness); and bugs or ticks can transmit Chagas disease, Lyme disease and encephalitis.
Together, the major vector-borne diseases kill more than 700 000 people each year, with populations in poverty-stricken tropical and subtropical areas at highest risk. Other vector-borne diseases, such as tick-borne encephalitis, are of increasing concern in temperate regions.
Rapid unplanned urbanization, massive increases in international travel and trade, altered agricultural practices and other environmental changes are fuelling the spread of vectors worldwide, putting more and more people at risk. Malnourished people and those with weakened immunity are especially susceptible.
A new approach
Over the past year, WHO has spearheaded a new strategic approach to reprioritize vector control. The Global Malaria Programme and the Department of Control of Neglected Tropical Diseases – along with the Special Programme for Research and Training in Tropical Diseases, have led a broad consultation tapping into the experience of ministries of health and technical experts. The process was steered by a group of eminent scientists and public health experts led by Dr Santelli and Professor Thomas Scott from the Department of Entomology and Nematology at the University of California, Davis and resulted in the Global Vector Control Response (GVCR) 2017–2030.
At its Seventieth session, the World Health Assembly unanimously welcomed the proposed response.
The GVCR outlines key areas of activity that will radically change the control of vector-borne diseases:
- Aligning action across sectors, since vector control is more than just spraying insecticides or delivering nets. That might mean ministries of health working with city planners to eradicate breeding sites used by mosquitoes;
- Engaging and mobilizing communities to protect themselves and build resilience against future disease outbreaks;
- Enhancing surveillance to trigger early responses to increases in disease or vector populations, and to identify when and why interventions are not working as expected; and
- Scaling-up vector-control tools and using them in combination to maximize impact on disease while minimizing impact on the environment.
Specifically, the new integrated approach calls for national programmes to be realigned so that public health workers can focus on the complete spectrum of relevant vectors and thereby control all of the diseases they cause.
Recognizing that efforts must be adapted to local needs and sustained, the success of the response will depend on the ability of countries to strengthen their vector-control programmes with financial resources and staff.
A call to pursue novel interventions aggressively
The GVCR also calls for the aggressive pursuit of promising novel interventions such as devising new insecticides; creating spatial repellents and odour-baited traps; improving house screening; pursuing development of a common bacterium that stops viruses from replicating inside mosquitoes; and modifying the genes of male mosquitoes so that their offspring die early.
Economic development also brings solutions. “If people lived in houses that had solid floors and windows with screens or air conditioning, they wouldn’t need a bednet,” said Professor Scott. “So, by improving people’s standard of living, we would significantly reduce these diseases.”

The call for a more coherent and holistic approach to vector control does not diminish the considerable advances made against individual vector-borne diseases.
Malaria is a prime example. Over the past 15 years, its incidence in sub-Saharan Africa has been cut by 45% – primarily due to the massive use of insecticide-treated bed nets and spraying of residual insecticides inside houses.
But that success has had a down side.
“We’ve been so successful, in some ways, with our control that we reduced the number of public health entomologists – the people who can do this stuff well,” said Professor Steve Lindsay, a public health entomologist at Durham University in Britain. “We’re a disappearing breed.”
The GVCR calls for countries to invest in a vector-control workforce trained in public health entomology and empowered in health care responses.
“We now need more nuanced control – not one-size-fits-all, but to tailor control to local conditions,” Professor Lindsay said. This is needed to tackle new and emerging diseases, but also to push towards elimination of others such as malaria, he said.
Dr Lindsay noted that, under the new strategic approach, individual diseases such as Zika, dengue and chikungunya will no longer be considered as separate threats. “What this represents is not three different diseases, but one mosquito – Aedes aegypti,” said Professor Lindsay.
GVCR dovetails with Sustainable Development Goals
The GVCR will also help countries achieve at least 6 of the 17 Sustainable Development Goals. Of direct relevance are goal 3 on good health and well-being, goal 6 on clean water and sanitation, and goal 11 on sustainable cities and communities.
The GVCR goals are ambitious – to reduce mortality from vector-borne diseases by at least 75% and incidence by at least 60% by 2030 – and to prevent epidemics in all countries.
The annual price tag is US$ 330 million globally, or about 5 cents per person – for workforce, coordination and surveillance costs. This is a modest additional investment in relation to insecticide-treated nets, indoor sprays and community-based activities, which usually exceed US$ 1 per person protected per year.
It also represents less than 10% of what is currently spent each year on strategies to control vectors that spread malaria, dengue and Chagas disease alone. Ultimately, the shift in focus to integrated and locally adapted vector control will save money.
‘A call for action’
Dr Santelli expressed optimism that the GVCR will help ministries of health around the world gain support from their governments for a renewed focus on vector control.
“Most of all, this document is a call for action,” said Dr Santelli, who now serves as deputy director for epidemiology in the Brasilia office of the U.S. Centers for Disease Control and Prevention.
It will not be easy, she predicts. The work to integrate vector-control efforts across different diseases will require more equipment, more people and more money as well as a change in mentality. “The risk of inaction is greater,” said Dr Santelli, “given the growing number of emerging disease threats.” The potential impact of the GVCR is immense: to put in place new strategies that will reduce overall burden and, in some places, even eliminate these diseases once and for all.
Outcomes of Pregnancies with Laboratory Evidence of Possible Zika Virus Infection in the United States
Friday, June 2nd, 2017Outcomes for Completed Pregnancies in the US States and District of Columbia, 2016-2017
Completed pregnancies with or without birth defects: 1,579
Includes aggregated data reported to the US Zika Pregnancy Registry*
Liveborn infants with birth defects*: 72
Includes aggregated data reported to the US Zika Pregnancy Registry(https://www.cdc.gov/zika/hc-providers/registry.html)*
Pregnancy losses with birth defects**: 8
Includes aggregated data reported to the US Zika Pregnancy Registry(https://www.cdc.gov/zika/hc-providers/registry.html)*
*As of May 23, 2017
What these numbers show
- The number of completed pregnancies with or without birth defects include those that ended in a live birth, miscarriage, stillbirth, or termination.
- The number of liveborn infants and pregnancy losses with birth defects include those among completed pregnancies with laboratory evidence of possible Zika virus infection that have been reported to the US Zika Pregnancy Registry.
- These numbers rely on reporting to the US Zika Pregnancy Registry and may increase or decrease as new cases are added or information on existing cases is clarified. For example, CDC cannot report the number of completed pregnancies with or without poor pregnancy outcomes that have not yet been reported to the US Zika Pregnancy Registry.
- The number of liveborn infants and pregnancy losses with birth defects are combined for the 50 US states, and the District of Columbia. CDC is not reporting individual state, tribal, territorial or jurisdictional level data to protect the privacy of the women and children affected by Zika. CDC is using a consistent case inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes potentially related to Zika virus infection during pregnancy in the US states and territories. Puerto Rico is not using the same inclusion criteria; CDC is not reporting numbers for adverse pregnancy outcomes in the territories at this time.
- Birth defects reported include those that have been detected in infants infected with Zika before, during, or shortly after birth, including microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from damage to brain that affects nerves, muscles and bones, such as clubfoot or inflexible joints, and confirmed hearing loss.
What these new numbers do not show
- These numbers are not real time estimates. They reflect the outcomes of pregnancies with any laboratory evidence of possible Zika virus infection reported to the US Zika Pregnancy Registry as of 12 noon Tuesday the week prior. Additionally, there may be delays in reporting of pregnancy outcomes from the jurisdictions.
- Although these outcomes occurred in pregnancies with laboratory evidence of possible Zika virus infection, we do not know whether they were caused by Zika virus infection or other factors.
Where do these numbers come from?
- These data reflect pregnancies reported to the US Zika Pregnancy Registry(https://www.cdc.gov/zika/hc-providers/registry.html). CDC, in collaboration with state, local, tribal and territorial health departments, established this system for comprehensive monitoring of pregnancy and infant outcomes following Zika virus infection.
- The data collected through this system will be used to update recommendations for clinical care, to plan for services and support for pregnant women and families affected by Zika virus, and to improve prevention of Zika virus infection during pregnancy.
The US Zika Pregnancy Registry and the Puerto Rico Zika Active Pregnancy Surveillance System are covered by an assurance of confidentiality(https://www.cdc.gov/od/science/integrity/confidentiality/index.htm). This protection requires us to safeguard the information collected for the pregnant women and infants in the registries.
Detailed case inclusion criteria for healthcare providers
The following details the inclusion criteria for brain abnormalities and other adverse outcomes potentially related to Zika virus infection during pregnancy. All pregnancy outcomes are monitored, but weekly reporting of adverse outcomes is limited to those meeting the criteria below. All prenatal and postnatal adverse outcomes are reported for both Zika Pregnancy Registries (US Zika Pregnancy Registry, Zika Active Pregnancy Surveillance System) and Active Birth Defects Surveillance; however, case finding methods dictate some differences in specific case definitions.
Brain abnormalities with and without microcephaly
- Confirmed or possible congenital microcephaly#
- Intracranial calcifications
- Cerebral atrophy
- Abnormal cortical formation (e.g., polymicrogyria, lissencephaly, pachygyria, schizencephaly, gray matter heterotopia)
- Corpus callosum abnormalities
- Cerebellar abnormalities
- Porencephaly
- Hydranencephaly
- Ventriculomegaly / hydrocephaly (excluding “mild” ventriculomegaly without other brain abnormalities)
- Fetal brain disruption sequence (collapsed skull, overlapping sutures, prominent occipital bone, scalp rugae)
- Other major brain abnormalities, including intraventricular hemorrhage in utero (excluding post-natal IVH)
Neural tube defects and other early brain malformations
- Neural tube defects (NTD)
- Anencephaly / Acrania
- Encephalocele
- Spina bifida
- Holoprosencephaly / Arhinencephaly
Structural eye abnormalities
- Microphthalmia / Anophthalmia
- Coloboma
- Cataract
- Intraocular calcifications
- Chorioretinal anomalies involving the macula (e.g., chorioretinal atrophy and scarring, macular pallor, gross pigmentary mottling and retinal hemorrhage); excluding retinopathy of prematurity
- Optic nerve atrophy, pallor, and other optic nerve abnormalities
Consequences of central nervous system (CNS) dysfunction
- Congenital contractures (e.g., arthrogryposis, club foot, congenital hip dysplasia) with associated brain abnormalities
- Congenital deafness documented by postnatal testing
#Live births: measured head circumference (HC) adjusted for gestational age and sex <3rd percentile at birth, or if not measured at birth, within first 2 weeks of life; pregnancy loss: prenatal HC* more than 3 SD below the mean based on ultrasound or postnatal HC <3rd percentile. Birth measurements based on intergrowth21 standards which are based on measurements within 24 hours of birth, and therefore measurements within 24 hours of birth are appropriate for this assessment.
People infected with the outbreak strains of Salmonella, by state of residence, as of May 25, 2017 (n=372)
Friday, June 2nd, 2017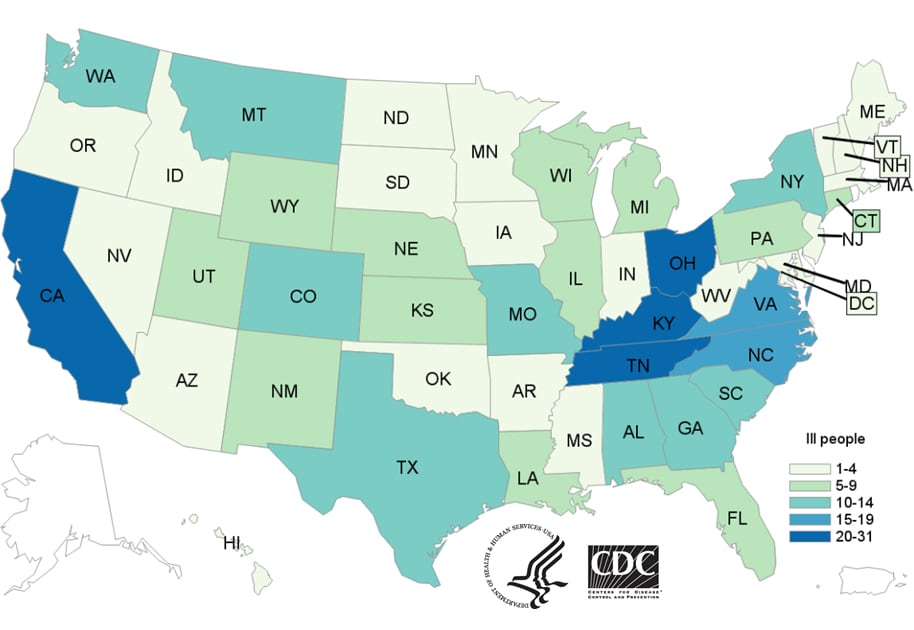
Posted June 1, 2017 2:45PM ET
Outbreak Advisory
| 8 Outbreaks |
372 Cases |
47 States |
71 Hospitalizations |
|---|
- CDC, many state departments of health and agriculture, and the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service are investigating eight multistate outbreaks of human Salmonella infections linked to contact with live poultry in backyard flocks.
- These outbreaks are caused by several kinds of Salmonella bacteria: Salmonella Braenderup, Salmonella Enteritidis, Salmonella Hadar, Salmonella I 4,[5],12:i-, Salmonella Indiana, Salmonella Infantis, Salmonella Mbandaka, and Salmonella Typhimurium.
- As of May 25, 2017, 372 people infected with the outbreak strains of Salmonella have been reported from 47 states.
- Illnesses started on dates ranging from January 4, 2017 to May 13, 2017.
- 71 ill people have been hospitalized, and no deaths have been reported.
- 36% of ill people are children younger than 5 years.
- Epidemiologic, traceback, and laboratory findings link the eight outbreaks to contact with live poultry, such as chicks and ducklings, which come from several hatcheries.
- In interviews, 190 (83%) of 228 ill people reported contact with live poultry in the week before illness started.
- People reported purchasing live baby poultry from several sources, including feed supply stores, websites, hatcheries, and from relatives.
- Contact with live poultry and the areas where they live and roam can make people sick with Salmonella infections. Chicks, ducklings, and other live poultry that look healthy and clean can still carry Salmonella bacteria.
- Outbreaks(https://www.cdc.gov/eid/article/22/10/15-0765_article) linked to contact with live poultry have increased in recent years as more people keep backyard flocks. In 2016(https://www.cdc.gov/salmonella/live-poultry-05-16/), a record number of illnesses were linked to contact with backyard poultry.





