Nipah in India: The first recorded NiV outbreak in South India.
December 9th, 2018Outbreak Investigation of Nipah Virus Disease in Kerala, India, 2018
“…..Results: During 2–29 May 2018, 23 cases were identified, including the index case; 18 were laboratory confirmed. The lineage of the NiV responsible for this outbreak was closer to the Bangladesh lineage. The median age of cases was 45 years; the sex of 15 (65%) was male. The median incubation period was 9.5 days (range, 6–14 days). Of the 23 cases, 20 (87%) had respiratory symptoms. The case-fatality rate was 91%; 2 cases survived. Risk factors for infection included close proximity (ie, touching, feeding, or nursing a NiV-infected person), enabling exposure to droplet infection. The public health response included isolation of cases, contact tracing, and enforcement of hospital infection control practices.
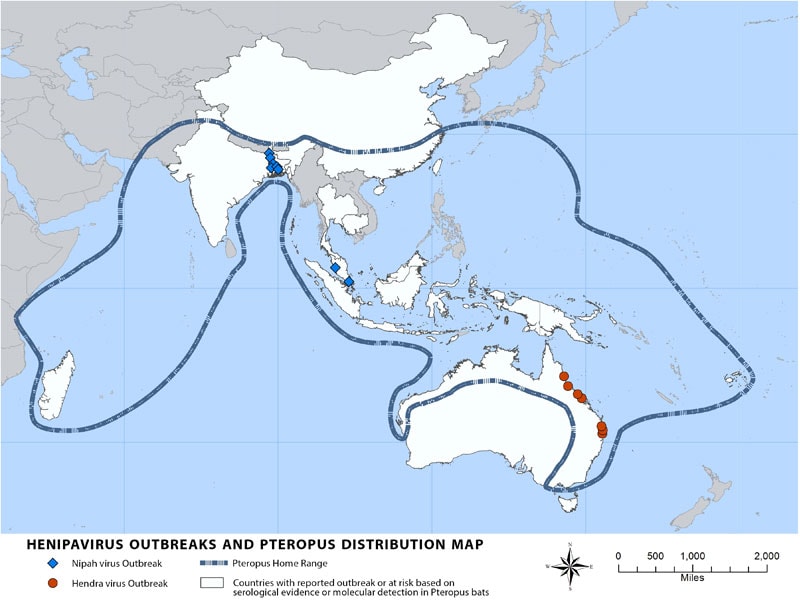
“Nipah virus (NiV) is a member of the family Paramyxoviridae, genus Henipavirus. NiV was initially isolated and identified in 1999 during an outbreak of encephalitis and respiratory illness among pig farmers and people with close contact with pigs in Malaysia and Singapore. Its name originated from Sungai Nipah, a village in the Malaysian Peninsula where pig farmers became ill with encephalitis. Given the relatedness of NiV to Hendra virus, bat species were quickly singled out for investigation and flying foxes of the genus Pteropus were subsequently identified as the reservoir for NiV….”
Transmission

Transmission of Nipah virus to humans may occur after direct contact with infected bats, infected pigs, or from other NiV infected people.
In Malaysia and Singapore, humans were apparently infected with Nipah virus only through close contact with infected pigs. The NiV strain identified in this outbreak appeared to have been transmitted initially from bats to pigs, with subsequent spread within pig populations. Incidental human infections resulted after exposure to infected pigs. No occurrence of person-to-person transmission was reported in this outbreak.
Conversely, person-to-person transmission of Nipah virus in Bangladesh and India is regularly reported. This is most commonly seen in the family and caregivers of Nipah virus-infected patients. Transmission also occurs from direct exposure to infected bats. A common example is consumption of raw date palm sap contaminated with infectious bat excretions.
Signs and Symptoms
During the Nipah virus disease outbreak in 1998-99, 265 patients were infected with the virus. About 40% of those patients who entered hospitals with serious nervous disease died from the illness.
Long-term sequelae following Nipah virus infection have been noted, including persistent convulsions and personality changes.
Latent infections with subsequent reactivation of Nipah virus and death have also been reported months and even years after exposure.
Treatment
Treatment is limited to supportive care. Because Nipah virus encephalitis can be transmitted person-to-person, standard infection control practices and proper barrier nursing techniques are important in preventing hospital-acquired infections (nosocomial transmission).
The drug ribavirin has been shown to be effective against the viruses in vitro, but human investigations to date have been inconclusive and the clinical usefulness of ribavirin remains uncertain.
Passive immunization using a human monoclonal antibody targeting the Nipah G glycoprotein has been evaluated in the post-exposure therapy in the ferret model and found to be of benefit.

