Archive for the ‘Zika virus’ Category
WHO: New vector control response seen as game-changer
Friday, June 2nd, 2017The call came from the WHO Director-General in May 2016 for a renewed attack on the global spread of vector-borne diseases.
“What we are seeing now looks more and more like a dramatic resurgence of the threat from emerging and re-emerging infectious diseases,” Dr Margaret Chan told Member States at the Sixty-ninth World Health Assembly. “The world is not prepared to cope.”
Dr Chan noted that the spread of Zika virus disease, the resurgence of dengue, and the emerging threat from chikungunya were the result of weak mosquito control policies from the 1970s. It was during that decade that funding and efforts for vector control were greatly reduced.
‘Vector control has not been a priority’
Dr Ana Carolina Silva Santelli has witnessed this first-hand. As former head of the programme for malaria, dengue, Zika and chikungunya with Brazil’s Ministry of Health, she saw vector-control efforts wane over her 13 years there. Equipment such as spraying machines, supplies such as insecticides and personnel such as entomologists were not replaced as needed. “Vector control has not been a priority,” she said.
Today more than 80% of the world’s population is at risk of vector-borne disease, with half at risk of two or more diseases. Mosquitoes can transmit, among other diseases, malaria, lymphatic filariasis, Japanese encephalitis and West Nile; flies can transmit onchocerciasis, leishmaniasis and human African trypanosomiasis (sleeping sickness); and bugs or ticks can transmit Chagas disease, Lyme disease and encephalitis.
Together, the major vector-borne diseases kill more than 700 000 people each year, with populations in poverty-stricken tropical and subtropical areas at highest risk. Other vector-borne diseases, such as tick-borne encephalitis, are of increasing concern in temperate regions.
Rapid unplanned urbanization, massive increases in international travel and trade, altered agricultural practices and other environmental changes are fuelling the spread of vectors worldwide, putting more and more people at risk. Malnourished people and those with weakened immunity are especially susceptible.
A new approach
Over the past year, WHO has spearheaded a new strategic approach to reprioritize vector control. The Global Malaria Programme and the Department of Control of Neglected Tropical Diseases – along with the Special Programme for Research and Training in Tropical Diseases, have led a broad consultation tapping into the experience of ministries of health and technical experts. The process was steered by a group of eminent scientists and public health experts led by Dr Santelli and Professor Thomas Scott from the Department of Entomology and Nematology at the University of California, Davis and resulted in the Global Vector Control Response (GVCR) 2017–2030.
At its Seventieth session, the World Health Assembly unanimously welcomed the proposed response.
The GVCR outlines key areas of activity that will radically change the control of vector-borne diseases:
- Aligning action across sectors, since vector control is more than just spraying insecticides or delivering nets. That might mean ministries of health working with city planners to eradicate breeding sites used by mosquitoes;
- Engaging and mobilizing communities to protect themselves and build resilience against future disease outbreaks;
- Enhancing surveillance to trigger early responses to increases in disease or vector populations, and to identify when and why interventions are not working as expected; and
- Scaling-up vector-control tools and using them in combination to maximize impact on disease while minimizing impact on the environment.
Specifically, the new integrated approach calls for national programmes to be realigned so that public health workers can focus on the complete spectrum of relevant vectors and thereby control all of the diseases they cause.
Recognizing that efforts must be adapted to local needs and sustained, the success of the response will depend on the ability of countries to strengthen their vector-control programmes with financial resources and staff.
A call to pursue novel interventions aggressively
The GVCR also calls for the aggressive pursuit of promising novel interventions such as devising new insecticides; creating spatial repellents and odour-baited traps; improving house screening; pursuing development of a common bacterium that stops viruses from replicating inside mosquitoes; and modifying the genes of male mosquitoes so that their offspring die early.
Economic development also brings solutions. “If people lived in houses that had solid floors and windows with screens or air conditioning, they wouldn’t need a bednet,” said Professor Scott. “So, by improving people’s standard of living, we would significantly reduce these diseases.”

The call for a more coherent and holistic approach to vector control does not diminish the considerable advances made against individual vector-borne diseases.
Malaria is a prime example. Over the past 15 years, its incidence in sub-Saharan Africa has been cut by 45% – primarily due to the massive use of insecticide-treated bed nets and spraying of residual insecticides inside houses.
But that success has had a down side.
“We’ve been so successful, in some ways, with our control that we reduced the number of public health entomologists – the people who can do this stuff well,” said Professor Steve Lindsay, a public health entomologist at Durham University in Britain. “We’re a disappearing breed.”
The GVCR calls for countries to invest in a vector-control workforce trained in public health entomology and empowered in health care responses.
“We now need more nuanced control – not one-size-fits-all, but to tailor control to local conditions,” Professor Lindsay said. This is needed to tackle new and emerging diseases, but also to push towards elimination of others such as malaria, he said.
Dr Lindsay noted that, under the new strategic approach, individual diseases such as Zika, dengue and chikungunya will no longer be considered as separate threats. “What this represents is not three different diseases, but one mosquito – Aedes aegypti,” said Professor Lindsay.
GVCR dovetails with Sustainable Development Goals
The GVCR will also help countries achieve at least 6 of the 17 Sustainable Development Goals. Of direct relevance are goal 3 on good health and well-being, goal 6 on clean water and sanitation, and goal 11 on sustainable cities and communities.
The GVCR goals are ambitious – to reduce mortality from vector-borne diseases by at least 75% and incidence by at least 60% by 2030 – and to prevent epidemics in all countries.
The annual price tag is US$ 330 million globally, or about 5 cents per person – for workforce, coordination and surveillance costs. This is a modest additional investment in relation to insecticide-treated nets, indoor sprays and community-based activities, which usually exceed US$ 1 per person protected per year.
It also represents less than 10% of what is currently spent each year on strategies to control vectors that spread malaria, dengue and Chagas disease alone. Ultimately, the shift in focus to integrated and locally adapted vector control will save money.
‘A call for action’
Dr Santelli expressed optimism that the GVCR will help ministries of health around the world gain support from their governments for a renewed focus on vector control.
“Most of all, this document is a call for action,” said Dr Santelli, who now serves as deputy director for epidemiology in the Brasilia office of the U.S. Centers for Disease Control and Prevention.
It will not be easy, she predicts. The work to integrate vector-control efforts across different diseases will require more equipment, more people and more money as well as a change in mentality. “The risk of inaction is greater,” said Dr Santelli, “given the growing number of emerging disease threats.” The potential impact of the GVCR is immense: to put in place new strategies that will reduce overall burden and, in some places, even eliminate these diseases once and for all.
Outcomes of Pregnancies with Laboratory Evidence of Possible Zika Virus Infection in the United States
Friday, June 2nd, 2017Outcomes for Completed Pregnancies in the US States and District of Columbia, 2016-2017
Completed pregnancies with or without birth defects: 1,579
Includes aggregated data reported to the US Zika Pregnancy Registry*
Liveborn infants with birth defects*: 72
Includes aggregated data reported to the US Zika Pregnancy Registry(https://www.cdc.gov/zika/hc-providers/registry.html)*
Pregnancy losses with birth defects**: 8
Includes aggregated data reported to the US Zika Pregnancy Registry(https://www.cdc.gov/zika/hc-providers/registry.html)*
*As of May 23, 2017
What these numbers show
- The number of completed pregnancies with or without birth defects include those that ended in a live birth, miscarriage, stillbirth, or termination.
- The number of liveborn infants and pregnancy losses with birth defects include those among completed pregnancies with laboratory evidence of possible Zika virus infection that have been reported to the US Zika Pregnancy Registry.
- These numbers rely on reporting to the US Zika Pregnancy Registry and may increase or decrease as new cases are added or information on existing cases is clarified. For example, CDC cannot report the number of completed pregnancies with or without poor pregnancy outcomes that have not yet been reported to the US Zika Pregnancy Registry.
- The number of liveborn infants and pregnancy losses with birth defects are combined for the 50 US states, and the District of Columbia. CDC is not reporting individual state, tribal, territorial or jurisdictional level data to protect the privacy of the women and children affected by Zika. CDC is using a consistent case inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes potentially related to Zika virus infection during pregnancy in the US states and territories. Puerto Rico is not using the same inclusion criteria; CDC is not reporting numbers for adverse pregnancy outcomes in the territories at this time.
- Birth defects reported include those that have been detected in infants infected with Zika before, during, or shortly after birth, including microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from damage to brain that affects nerves, muscles and bones, such as clubfoot or inflexible joints, and confirmed hearing loss.
What these new numbers do not show
- These numbers are not real time estimates. They reflect the outcomes of pregnancies with any laboratory evidence of possible Zika virus infection reported to the US Zika Pregnancy Registry as of 12 noon Tuesday the week prior. Additionally, there may be delays in reporting of pregnancy outcomes from the jurisdictions.
- Although these outcomes occurred in pregnancies with laboratory evidence of possible Zika virus infection, we do not know whether they were caused by Zika virus infection or other factors.
Where do these numbers come from?
- These data reflect pregnancies reported to the US Zika Pregnancy Registry(https://www.cdc.gov/zika/hc-providers/registry.html). CDC, in collaboration with state, local, tribal and territorial health departments, established this system for comprehensive monitoring of pregnancy and infant outcomes following Zika virus infection.
- The data collected through this system will be used to update recommendations for clinical care, to plan for services and support for pregnant women and families affected by Zika virus, and to improve prevention of Zika virus infection during pregnancy.
The US Zika Pregnancy Registry and the Puerto Rico Zika Active Pregnancy Surveillance System are covered by an assurance of confidentiality(https://www.cdc.gov/od/science/integrity/confidentiality/index.htm). This protection requires us to safeguard the information collected for the pregnant women and infants in the registries.
Detailed case inclusion criteria for healthcare providers
The following details the inclusion criteria for brain abnormalities and other adverse outcomes potentially related to Zika virus infection during pregnancy. All pregnancy outcomes are monitored, but weekly reporting of adverse outcomes is limited to those meeting the criteria below. All prenatal and postnatal adverse outcomes are reported for both Zika Pregnancy Registries (US Zika Pregnancy Registry, Zika Active Pregnancy Surveillance System) and Active Birth Defects Surveillance; however, case finding methods dictate some differences in specific case definitions.
Brain abnormalities with and without microcephaly
- Confirmed or possible congenital microcephaly#
- Intracranial calcifications
- Cerebral atrophy
- Abnormal cortical formation (e.g., polymicrogyria, lissencephaly, pachygyria, schizencephaly, gray matter heterotopia)
- Corpus callosum abnormalities
- Cerebellar abnormalities
- Porencephaly
- Hydranencephaly
- Ventriculomegaly / hydrocephaly (excluding “mild” ventriculomegaly without other brain abnormalities)
- Fetal brain disruption sequence (collapsed skull, overlapping sutures, prominent occipital bone, scalp rugae)
- Other major brain abnormalities, including intraventricular hemorrhage in utero (excluding post-natal IVH)
Neural tube defects and other early brain malformations
- Neural tube defects (NTD)
- Anencephaly / Acrania
- Encephalocele
- Spina bifida
- Holoprosencephaly / Arhinencephaly
Structural eye abnormalities
- Microphthalmia / Anophthalmia
- Coloboma
- Cataract
- Intraocular calcifications
- Chorioretinal anomalies involving the macula (e.g., chorioretinal atrophy and scarring, macular pallor, gross pigmentary mottling and retinal hemorrhage); excluding retinopathy of prematurity
- Optic nerve atrophy, pallor, and other optic nerve abnormalities
Consequences of central nervous system (CNS) dysfunction
- Congenital contractures (e.g., arthrogryposis, club foot, congenital hip dysplasia) with associated brain abnormalities
- Congenital deafness documented by postnatal testing
#Live births: measured head circumference (HC) adjusted for gestational age and sex <3rd percentile at birth, or if not measured at birth, within first 2 weeks of life; pregnancy loss: prenatal HC* more than 3 SD below the mean based on ultrasound or postnatal HC <3rd percentile. Birth measurements based on intergrowth21 standards which are based on measurements within 24 hours of birth, and therefore measurements within 24 hours of birth are appropriate for this assessment.
First time: Zika virus infection – India
Sunday, May 28th, 2017On 15 May 2017, the Ministry of Health and Family Welfare-Government of India (MoHFW) reported three laboratory-confirmed cases of Zika virus disease in Bapunagar area, Ahmedabad District, Gujarat, State, India.
The routine laboratory surveillance detected a laboratory-confirmed case of Zika virus disease through RT-PCR test at B.J. Medical College, Ahmedabad, Gujarat. The etiology of this case has been further confirmed through a positive RT-PCR test and sequencing at the national reference laboratory, National Institute of Virology (NIV), Pune on 4 January 2017 (case 2, below). Two additional cases (case 1 and case 3), have then been identified through the Acute Febrile Illness (AFI) and the Antenatal clinic (ANC) surveillance. The cases are reported below in chronological order:
- Case 1: During the Acute Febrile Illness (AFI) surveillance between 10 to 16 February 2016, a total of 93 blood samples were collected at BJ Medical College (BJMC), Ahmedabad, Gujarat State. One sample from a 64-year-old male presenting with febrile illness of 8 days’ duration (negative for dengue infection) was found to be positive for Zika virus at BJMC, Ahmedabad. This is the first Zika positive case reported through AFI surveillance at BJMC, Ahmedabad, Gujarat State.
- Case 2: A 34-year-old female, delivered a clinically well baby at BJMC in Ahmedabad on 9 November 2016. During her hospital stay, she developed a low grade fever after delivery. No history of fever during pregnancy and no history of travel for the past three months was reported. A sample from the patient was referred to the Viral Research & Diagnostic Laboratory (VRDL) at the BJMC for dengue testing and thereafter found to be positive for Zika virus. She was discharged after one week (on 16 November 2016). The sample was re-confirmed as Zika virus positive by RT-PCR and sequencing at NIV, Pune.
- Case 3: During the Antenatal clinic (ANC) surveillance between 6 and 12 January 2017, a total of 111 blood samples were collected at BJMC. One sample from a 22-year-old pregnant female in her 37th week of pregnancy has been tested positive for Zika virus disease.
Public health response
- National Guidelines and Action Plan on Zika virus disease have been shared with the States to prevent an outbreak of Zika virus disease and containment of spread in case of any outbreak.
- An Inter-Ministerial Task Force has been set up under the Chairmanship of Secretary (Health and Family Welfare) together with Secretary (Bio-Technology), and Secretary (Department of Health Research). The Joint Monitoring Group, a technical group tasked to monitor emerging and re-emerging diseases is regularly reviewing the global situation on Zika virus disease.
- All the international airports and ports have displayed information for travellers on Zika virus disease.
- The airport health officers along with airport organizations, National Centre for Disease Control, and the National Vector Borne Disease Control Programme are monitoring appropriate vector control measures in airport premises.
- The Integrated Disease Surveillance Programme (IDSP) is tracking for clustering of acute febrile illness in the community.
- In addition to National Institute of Virology, Pune, and NCDC in Delhi, 25 laboratories have also been strengthened by Indian Council of Medical Research for laboratory diagnosis. In addition, 3 entomological laboratories are conducting Zika virus testing on mosquito samples.
- The Indian Council of Medical Research (ICMR) has tested 34 233 human samples and 12 647 mosquito samples for the presence of Zika virus. Among those, close to 500 mosquitos samples were collected from Bapunagar area, Ahmedabad District, in Gujarat, and were found negative for Zika.
- The Rashtriya Bal Swasthya Karyakram (RBSK) is monitoring microcephaly from 55 sentinel sites. As of now, no increase in number of cases or clustering of microcephaly has been reported from these centers.
- Risk communication materials are being finalized by the Central Health Education Bureau, in consultation with UNICEF.
WHO risk assessment
This report is important as it describes the first cases of Zika virus infections and provides evidence on the circulation of the virus in India. These findings suggest low level transmission of Zika virus and new cases may occur in the future. Efforts to strengthen surveillance should be maintained in order to better characterize the intensity of the viral circulation and geographical spread, and monitor Zika virus related complications. Zika virus is known to be circulating in South East Asia Region and these findings do not change the global risk assessment. WHO encourages Member states to report similar findings to better understand the global epidemiology of Zika virus.
The risk of further spread of Zika virus to areas where the competent vectors, the Aedes mosquitoes, are present is significant given the wide geographical distribution of these mosquitoes in various regions of the world. WHO continues to monitor the epidemiological situation and conduct risk assessment based on the latest available information.
WHO advice
Prevention and control relies on reducing mosquitoes through source reduction (removal and modification of breeding sites) and reducing contact between mosquitoes and people. During outbreaks, health authorities may advise that spraying of insecticides be carried out. Insecticides recommended by the WHO Pesticide Evaluation Scheme may also be used as larvicides to treat relatively large water containers.
Basic precautions for protection from mosquito bites should be taken by people traveling to high risk areas, especially pregnant women. These include use of repellents, wearing light colored, long sleeved shirts and pants and ensuring rooms are fitted with screens to prevent mosquitoes from entering.
WHO does not recommend any travel or trade restriction to India based on the current information available.
edes aegypti mosquitoes that carry Zika can also transmit dengue and chikungunya in the same bite.
Saturday, May 20th, 2017Rückert, C. et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun. 8, 15412 doi: 10.1038/ncomms15412 (2017).
“…..Thus, we here expose Ae. aegypti mosquitoes to chikungunya, dengue-2 or Zika viruses, both individually and as double and triple infections. Our results show that these mosquitoes can be infected with and can transmit all combinations of these viruses simultaneously. Importantly, infection, dissemination and transmission rates in mosquitoes are only mildly affected by coinfection…..”
A novel retinal sign that appears to be specific to Ebola survivors.
Sunday, May 14th, 2017Citation: Steptoe PJ, Scott JT, Baxter JM, Parkes CK, Dwivedi R, Czanner G, et al. Novel retinal lesion in Ebola survivors, Sierra Leone, 2016. Emerg Infect Dis. 2017 Jul [date cited]. https://dx.doi.org/10.3201/eid2307.161608
Abstract: We conducted a case–control study in Freetown, Sierra Leone, to investigate ocular signs in Ebola virus disease (EVD) survivors. A total of 82 EVD survivors with ocular symptoms and 105 controls from asymptomatic civilian and military personnel and symptomatic eye clinic attendees underwent ophthalmic examination, including widefield retinal imaging. Snellen visual acuity was <6/7.5 in 75.6% (97.5% CI 63%–85.7%) of EVD survivors and 75.5% (97.5% CI 59.1%–87.9%) of controls. Unilateral white cataracts were present in 7.4% (97.5% CI 2.4%–16.7%) of EVD survivors and no controls. Aqueous humor from 2 EVD survivors with cataract but no anterior chamber inflammation were PCR-negative for Zaire Ebola virus, permitting cataract surgery. A novel retinal lesion following the anatomic distribution of the optic nerve axons occurred in 14.6% (97.5% CI 7.1%–25.6%) of EVD survivors and no controls, suggesting neuronal transmission as a route of ocular entry.
Volume 23, Number 7—July 2017
Research
Novel Retinal Lesion in Ebola Survivors, Sierra Leone, 2016
Figure 2
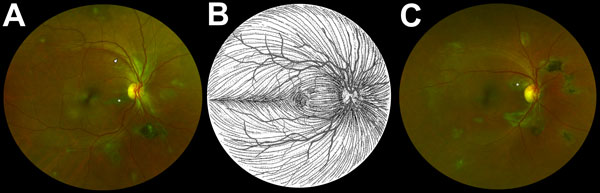
Figure 2. Composite scanning laser ophthalmoscope retinal images showing type 6 Ebola peripapillary or peripheral lesions, observed following the anatomic distribution of the ganglion cell axon (retinal nerve fiber layer), in a case–control study of ocular signs in Ebola virus disease survivors, Sierra Leone, 2016. A) Example 1, right eye. B) Illustration of the ganglion cell axon anatomic distribution. Courtesy of W.L.M. Alward. C) Example 2, right eye. Asterisks indicate curvilinear lesions distinct from the retinal vasculature. White arrowhead indicates retinal nerve fiber wedge defect.
Figure 3
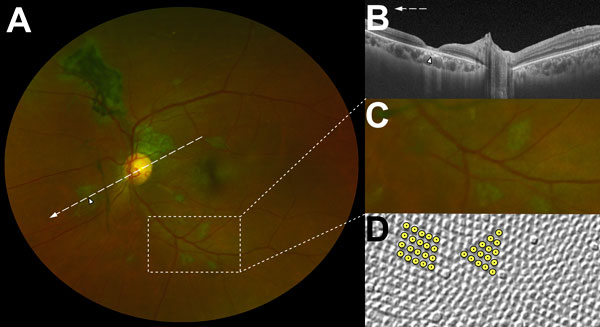
Figure 3. Characteristic features of lesions observed in a case–control study of ocular signs in Ebola virus disease survivors, Sierra Leone, 2016. A) Composite scanning laser ophthalmoscope retinal image, left eye. Arrow indicates direction of the optical coherence tomography scan. B) Optical coherence tomography. White, long, dashed line indicates cross-sectional plane; white arrowhead indicates Ebola lesion limited to the retinal layers with an intact retinal pigment epithelium. Magnified 1.5× from original image (panel A). C) Examples of straight-edged, sharp angulated lesions (magnified from panel A). D) Example of tangential section through the human fovea with illustrative highlighting of a triangular photoreceptor matrix corresponding to Ebola lesional shape. Courtesy of Ahnelt et al. (17).
Brazil has declared an end to a national emergency over the Zika virus after the number of cases dropped 95% between January and April, compared to the same period a year ago.
Friday, May 12th, 2017A novel virus-like particle (VLP) Zika vaccine elicited high titers of virus-neutralizing antibodies in mice.
Thursday, May 11th, 2017“…..Here we describe a novel strategy to assemble Zika virus-like particles (VLPs) by co-expressing the structural (CprME) and non-structural (NS2B/NS3) proteins, and demonstrate their effectiveness as vaccines. VLPs are produced in a suspension culture of mammalian cells and self-assembled into particles closely resembling Zika viruses as shown by electron microscopy studies. We tested various VLP vaccines and compared them to analogous compositions of an inactivated Zika virus (In-ZIKV) used as a reference. VLP immunizations elicited high titers of antibodies, as did the In-ZIKV controls. However, in mice the VLP vaccine stimulated significantly higher virus neutralizing antibody titers than comparable formulations of the In-ZIKV vaccine. The serum neutralizing activity elicited by the VLP vaccine was enhanced using a higher VLP dose and with the addition of an adjuvant, reaching neutralizing titers greater than those detected in the serum of a patient who recovered from a Zika infection in Brazil in 2015. Discrepancies in neutralization levels between the VLP vaccine and the In-ZIKV suggest that chemical inactivation has deleterious effects on neutralizing epitopes within the E protein. This along with the inability of a VLP vaccine to cause infection makes it a preferable candidate for vaccine development….”
Zika virus IgM can persist beyond 12 weeks in a subset of infected people.
Saturday, May 6th, 2017Distributed via the CDC Health Alert Network
May 05, 2017 1130 ET (11:30 AM ET)
CDCHAN-00402
Prolonged IgM Antibody Response in People Infected with Zika Virus: Implications for Interpreting Serologic Testing Results for Pregnant Women
Summary
In July 2016, CDC issued Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure – United States, July 2016 (https://www.cdc.gov/mmwr/volumes/65/wr/mm6529e1.htm) that includes Zika virus immunoglobulin M (IgM) testing of pregnant women. However, some flavivirus infections can result in prolonged IgM responses (>12 weeks) that make it difficult to determine the timing of infection, especially in testing of asymptomatic people. Emerging epidemiologic and laboratory data indicate that Zika virus IgM can persist beyond 12 weeks in a subset of infected people. Therefore, detection of IgM may not always indicate a recent infection. Although IgM persistence could affect IgM test interpretation for all infected people, it would have the greatest effect on clinical management of pregnant women with a history of living in or traveling to areas with Zika virus transmission. Pregnant women who test positive for IgM antibody may have been infected with Zika virus and developed an IgM response before conception.
For asymptomatic pregnant women with a history of living in or traveling to areas with Zika virus transmission, Zika virus nucleic acid test (NAT) testing at least once per trimester is recommended, in addition to IgM testing as previously recommended. If positive, this may provide a more definitive diagnosis of recent Zika infection. However, a negative NAT does not rule out recent infection because viral ribonucleic acid (RNA) declines over time. Other diagnostic methods, such as NAT testing of amniocentesis specimens or serial ultrasounds, may provide additional information to help determine whether the IgM test results suggest a recent infection. Providers should counsel women on the limitations of all tests. In addition, providers may wish to consider IgM testing as part of pre-conception counseling to establish baseline IgM results before pregnancy; however, preconception negative IgM results might have limited value for women at ongoing risk of Zika infection. NAT testing should be performed for any pregnant woman who becomes symptomatic or who has a sexual partner who tests positive for Zika virus infection.
Recommendations
For asymptomatic pregnant women with possible Zika virus exposure before conception, (particularly women who lived in or traveled to areas with posted CDC Zika Travel Notices https://wwwnc.cdc.gov/travel/page/zika-information), CDC recommends that healthcare providers take these steps:
- Screen pregnant women for risk of Zika exposure and symptoms of Zika. Promptly test pregnant women with NAT if they become symptomatic during their pregnancy or if a sexual partner tests positive for Zika virus infection.
- Conduct NAT testing at least once per trimester, unless a previous test has been positive.*
- Consider NAT testing of amniocentesis specimens if amniocentesis is performed for other reasons.†
- Counsel pregnant women each trimester on the limitations of IgM and NAT testing. For more information about Zika virus testing, see: https://www.cdc.gov/zika/pdfs/living_in.pdf. For more information about counseling before testing, see: https://www.cdc.gov/zika/pdfs/pretestingcounselingscript_livingin.pdf.
- Consider IgM testing to determine baseline Zika virus IgM levels as part of preconception counseling.§ For more information about preconception counseling, see: https://www.cdc.gov/zika/pdfs/preconception-counseling.pdf
Recommendations for testing symptomatic pregnant women, remain unchanged (https://www.cdc.gov/mmwr/volumes/65/wr/mm6529e1.htm). However, if a symptomatic pregnant woman is IgM positive and NAT negative, and lived in or traveled to an area with a posted CDC Zika Travel Notice (https://wwwnc.cdc.gov/travel/page/zika-information), healthcare providers should recognize that the positive IgM result does not necessarily indicate recent infection.
CDC will update clinical management (https://www.cdc.gov/mmwr/volumes/65/wr/mm6529e1.htm) and laboratory testing (https://www.cdc.gov/zika/laboratories/lab-guidance.html) recommendations as new information becomes available.
Background
Some flavivirus infections have been reported to result in prolonged IgM responses that make it difficult to differentiate recent from prior infections in areas with ongoing transmission. For dengue virus, IgM was determined to persist for 6 months (179 days [95% confidence interval, 155 to 215 days]) for primary infections and 4.6 months (139 days [95% confidence interval, 119 to 167 days]) after infection with another flavivirus1. IgM antibodies against West Nile virus, another flavivirus related to Zika virus, have been detected in asymptomatic, infected blood donors for at least three months after they donated blood, and almost half of tested patients with West Nile virus infection had serum IgM antibodies >1 year after infection2,3.
Recent findings suggest that Zika virus infection may also result in IgM persistence that may make it difficult to differentiate prior from recent infections. A recent study in Puerto Rico of symptomatic patients with NAT-confirmed Zika virus infection detected Zika virus IgM in 100% (28/28) of participants at 8 to 15 days after symptom onset, and 87% (52/60) at greater than 60 days after symptom onset4. Unpublished data on the symptomatic patients from this ongoing study show a median time to first negative Zika virus IgM as 4 months (122 days [range 8-210 days]). More data are needed to accurately estimate the proportion of persons who are likely to have Zika IgM persist beyond 12 weeks after infection.
IgM test results can also be difficult to interpret because of cross-reactivity with other flaviviruses, particularly dengue virus, when a person has been previously infected or vaccinated with a related flavivirus. During 2016, Puerto Rico had limited dengue virus transmission and, therefore, people who tested positive for Zika IgM antibody could be assumed to have had recent Zika virus infection. However, if dengue virus transmission were to increase, guidance for interpretation of Zika virus IgM testing results may need to be reconsidered.
NAT testing may be useful in testing pregnant women as an indicator of current infection and increased risk to the fetus. In the same study from Puerto Rico discussed above, viral RNA was detected in 36% (10/28) of participants at 8‒15 days after symptom onset, 21% (27/129) at 16‒30 days after symptom onset, and 4% (3/79) more than 60 days after symptom onset4. A limited number of studies have demonstrated detection of viral nucleic acid in some pregnant women for even longer periods after symptom onset. For example, three of the five pregnant women included in the study from Puerto Rico had detectable RNA at 46 days and one still had detectable RNA at 80 days after symptom onset4. In another case series, some pregnant women had Zika virus RNA detectable up to 107 days after symptom onset5.
For More Information
Update: Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure – United States, July 2016. MMWR Morb Mortal Wkly Rep. 2016 Jul 25;65(29):739-44. https://www.cdc.gov/mmwr/volumes/65/wr/mm6529e1.htm
Guidance for U.S. Laboratories Testing for Zika Virus Infection. https://www.cdc.gov/zika/laboratories/lab-guidance.html
Update: Interim Guidance for Preconception Counseling and Prevention of Sexual Transmission of Zika Virus for Persons with Possible Zika Virus Exposure – United States, September 2016. MMWR Morb Mortal Wkly Rep. 2016 Oct 7;65(39):1077-1081. https://www.cdc.gov/mmwr/volumes/65/wr/mm6539e1.htm?s_cid=mm6539e1_w
References
- Prince HE, Matud JL. Estimation of dengue virus IgM persistence using regression analysis. Clin Vaccine Immunol 2011;18: 2183-5.
- Roehrig JT, Nash D, Maldin B, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 2003;9:376-9. http:// dx.doi.org/10.3201/ eid0903.020531
- Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis 2008;198:984-93. http:// dx.doi.org/10.1086/591467
- Paz-Baily G, Rosenberg ES, Doyle K, et al. Persistence of Zika virus in body fluids— Preliminary report. N Engl J Rep 2017. DOI: 10.1056/NEJMoa1613108
- Suy A, Sulleiro E, Rodó C, et al. Prolonged Zika virus viremia during pregnancy. N Engl J Med. 2016;375:2611-2613.
- Reynolds MR, Jones AM, Petersen EE, et al. Vital signs: Update on Zika virus–associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure — U.S. Zika pregnancy registry, 2016. MMWR Morb Mortal Wkly Rep 2017;66:366-373.
Footnotes
* Birth defects have been reported in a higher proportion of fetuses or infants whose mothers were infected during the first trimester of pregnancy than in later trimesters. In pregnancies with symptom onset or exposure during the first trimester that were limited to those with laboratory-confirmed Zika virus infection, 15% of completed pregnancies had reported birth defects of the type seen with congenital Zika infection6.
† Consideration of amniocentesis should be individualized, because data about its usefulness in diagnosing congenital Zika virus infection are limited. The presence of Zika virus RNA in the amniotic fluid might indicate fetal infection; however, a negative result does not exclude congenital Zika virus infection
§ Preconceptional IgM testing is recommended to establish a baseline IgM level before pregnancy. However, given the limitations of interpreting IgM testing, the results of these tests should not be used to guide decisions about pregnancy timing for women living in areas with ongoing risk of transmission.
Echocardiography evaluation of a group of Brazilian babies with Zika-related birth defects found three times the expected rate of congenital heart disease (CHD), but only one infant had symptoms and most had minor septal defects that weren’t hemodynamically significant.
Saturday, April 22nd, 2017Cavalcanti DD, Alves LV, Furtado GJ, Santos CC, Feitosa FG, Ribeiro MC, et al. (2017) Echocardiographic findings in infants with presumed congenital Zika syndrome: Retrospective case series study. PLoS ONE 12(4): e0175065. https://doi.org/10.1371/journal.pone.0175065
Phase 2 Zika vaccine trial begins in U.S., Central and South America
Monday, April 3rd, 2017Friday, March 31, 2017
Study will evaluate NIH’s experimental DNA vaccine.
 A vial of the NIAID Zika Virus Investigational DNA Vaccine. NIAID
A vial of the NIAID Zika Virus Investigational DNA Vaccine. NIAIDVaccinations have begun in a multi-site Phase 2/2b clinical trial testing an experimental DNA vaccine designed to protect against disease caused by Zika infection. The vaccine was developed by government scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). NIAID is leading the trial, which aims to enroll at least 2,490 healthy participants in areas of confirmed or potential active mosquito-transmitted Zika infection, including the continental United States and Puerto Rico, Brazil, Peru, Costa Rica, Panama and Mexico. The two-part trial, called VRC 705, further evaluates the vaccine’s safety and ability to stimulate an immune response in participants, and assesses the optimal dose for administration. It also will attempt to determine if the vaccine can effectively prevent disease caused by Zika infection.
Most people with Zika infection have either no or only mild symptoms, such as fever, rash, joint pain and conjunctivitis (red eyes). However, when Zika infection occurs during pregnancy, the pregnant woman can pass the virus to her fetus, which can result in a range of fetal defects known collectively as congenital Zika syndrome. Currently there is no licensed vaccine to prevent disease caused by Zika infection, which is mainly transmitted via the bite of infected Aedes aegypti mosquitoes but also can be transmitted sexually.
“We are pleased to have advanced rapidly one of NIAID’s experimental Zika vaccines into this next stage of testing in volunteers. We expect this study will yield valuable insight into the vaccine’s safety and ability to prevent disease caused by Zika infection,” said NIAID Director Anthony S. Fauci, M.D. “A safe and effective Zika vaccine is urgently needed to prevent the often-devastating birth defects that can result from Zika virus infection during pregnancy. Evidence also is accumulating that Zika can cause a variety of health problems in adults as well. This trial marks a significant milestone in our efforts to develop countermeasures for a pandemic in progress.”
Scientists at NIAID’s Vaccine Research Center (VRC) developed the NIAID Zika virus investigational DNA vaccine. It entered early-stage human testing in 2016 following extensive testing in animal models. Initial findings indicate the vaccine is safe and able to induce a neutralizing antibody response against Zika virus. The Phase 2/2b trial aims to gain more safety and immune response data and determine if this immune response protects against disease caused by natural Zika infection.
The Zika vaccine platform is based on a strategy VRC scientists used previously to develop a West Nile virus vaccine candidate. The Zika vaccine candidate being tested in this study contains a small circular piece of DNA called a plasmid into which scientists have inserted genes that encode two proteins found on the surface of the Zika virus. Once injected into muscle, the encoded proteins assemble into particles that mimic Zika virus and trigger the body’s immune system to respond. The vaccine does not contain infectious material, so it cannot cause Zika infection.
The trial is being led by protocol co-chairs Julie E. Ledgerwood, D.O., chief of VRC’s Clinical Trials Program, and Grace L. Chen, M.D., deputy chief of the same program.
The trial consists of two studies: part A and part B. Part A will build on ongoing Phase 1 trials to further evaluate the vaccine’s safety and ability to stimulate an immune response, specifically in populations where Zika could be endemic. It will also help determine the optimal dose and injection sites for administration. Part A will enroll 90 healthy men and non-pregnant women ages 18-35 years at three sites in Houston, Miami and San Juan, Puerto Rico. All participants will receive the investigational vaccine intramuscularly at three separate clinic visits each four weeks apart. Participants will be randomly assigned to receive either a standard dose or a high dose of the investigational vaccine at all three visits, and will be followed for about 32 weeks total.
Part B of the trial will enroll at least 2,400 healthy men and non-pregnant women ages 15-35 years. This part of the trial aims to determine if the vaccine can effectively protect against Zika-related disease when someone is naturally exposed to the virus. Sites will include the three locations from part A (Houston, Miami and San Juan) as well as two additional sites in San Juan, two sites in Costa Rica, and one site each in Peru, Brazil, Panama and Mexico. Additional sites might be added in the future. Participants will be randomly assigned to receive either the investigational vaccine or a placebo at three separate clinic visits each four weeks apart. The trial is double-blind, meaning neither the study investigators nor the participants will know who receives the investigational vaccine.
Part B participants will be followed for nearly two years, during which time they will undergo assessments for adverse events and symptoms of Zika infection. Trial participants in both parts will be counseled on how to protect against Zika infection. Investigators will compare the rates of confirmed cases of Zika in the placebo group and the vaccinated group to determine if the investigational vaccine protects against disease caused by Zika infection.
Each site will have a principal investigator responsible for ensuring daily review of safety data as they become available. A protocol safety review team that includes the protocol chairs and other medical officers at NIAID will review safety data reports weekly. The NIAID Intramural Data and Safety Monitoring Board will also review cumulative study data at least twice per year. The study is currently expected to be completed by 2019.
For more information about the trial, visit Questions and Answers: VRC 705: Phase 2/2b Trial Testing the NIAID Zika Virus Investigational DNA Vaccine.
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®


