Archive for December, 2017
Beware of Snail Mail: 10 Signs of a Suspicious Package
Sunday, December 31st, 2017t is important for you to prepare and know how to identify a suspicious package and what you can do to stay safe.
- Look at the handwriting. Suspicious packages are often addressed by hand in all capital letters, or with cut-and-paste lettering.
- Pay attention to the return address. Suspicious packages often do not have a return address, or they may be postmarked from a city that does not match the return address.
- Note the postage. A package with excessive postage (more than was necessary for a package to reach its destination) should be treated as suspicious. Sometimes suspicious packages are delivered with no postage.
- Wrapping matters. If a package is unprofessionally wrapped with excessive packing material such as tape and/or string it should be treated as suspicious. It may also be labeled with restrictive endorsements – Fragile: Handle with Care, Rush: Do Note Delay, Personal, Confidential, or Do Not X-Ray.
- Use your senses. Be aware if the package has an unknown liquid or powder seeping through the wrapping or a strange odor. Do NOT sniff, taste, or touch the package or ask others to do the same.
- Hands off. Do not open the item or shake or empty the contents.
- Keep your distance. If you think you are dealing with a suspicious package, leave the room and close the door behind you. It is important to section off or isolate the package so other people do not enter the area.
- Don’t run away. Leaving the area could potentially spread dangerous or deadly materials to other locations, including your home. The authorities will determine if you need to undergo decontamination, medical treatment, or simply monitoring for any side-effects.
- Call 9-1-1. Use a land line to call 9-1-1. Do not use a cell phone or device that sends a signal because it could trigger an explosive device.
- Stay calm. Listen to your intuition and do not worry about embarrassment if you are wrong about a package being suspicious. It is always better to be safe than sorry.
References
- Ready Navy: Suspicious Packages
- Department of Homeland Security: Handling Suspicious Mail
Posted on December 14, 2017 by
CDC: About increased influenza A(H3N2) activity
Sunday, December 31st, 2017Seasonal Influenza A(H3N2) Activity and Antiviral Treatment of Patients with Influenza
Distributed via the CDC Health Alert Network
December 27, 2017, 1030 ET (10:30 AM ET)
CDCHAN-00409
Summary
The Centers for Disease Control and Prevention (CDC) is providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment.
Background
In the United States (U.S.), influenza activity has increased significantly over recent weeks with influenza A(H3N2) viruses predominating so far this season. In the past, A(H3N2) virus-predominant influenza seasons have been associated with more hospitalizations and deaths in persons aged 65 years and older and young children compared to other age groups. In addition, influenza vaccine effectiveness (VE) in general has been lower against A(H3N2) viruses than against influenza A(H1N1)pdm09 or influenza B viruses. Last season, VE against circulating influenza A(H3N2) viruses was estimated to be 32% in the U.S. CDC expects that VE could be similar this season, should the same A(H3N2) viruses continue to predominate. For this reason, in addition to influenza vaccination for prevention of influenza, the use of antiviral medications for treatment of influenza becomes even more important than usual. The neuraminidase inhibitor (NAI) antiviral medications are most effective in treating influenza and reducing complications when treatment is started early. Evidence from previous influenza seasons suggests that NAI antivirals are underutilized in outpatients and hospitalized patients with influenza who are recommended for treatment.
This CDC Health Advisory is being issued to—
- Remind clinicians that influenza should be high on their list of possible diagnoses for ill patients because influenza activity is increasing nationwide, and
- Advise clinicians that all hospitalized patients and all high-risk patients (either hospitalized or outpatient) with suspected influenza should be treated as soon as possible with a neuraminidase inhibitor antiviral. While antiviral drugs work best when treatment is started within 2 days of illness onset, clinical benefit has been observed even when treatment is initiated later.
Recommendations
1. CDC Antiviral Recommendations for the 2017–2018 Season
CDC recommends antiviral medications for treatment of influenza as an important adjunct to annual influenza vaccination. Treatment with neuraminidase inhibitors has been shown to have clinical and public health benefit in reducing illness and severe outcomes of influenza based on evidence from randomized controlled trials, meta-analyses of randomized controlled trials, and observational studies during past influenza seasons and during the 2009 H1N1 pandemic.1,2,3,4,5,6
2. All Hospitalized, Severely Ill, and High-Risk Patients with Suspected or Confirmed Influenza Should Be Treated with Antivirals
Any patient with suspected or confirmed influenza in the following categories should be treated as soon as possible with a neuraminidase inhibitor:
1) Any patient who is hospitalized—treatment is recommended for all hospitalized patients;
2) Any patient who has severe, complicated, or progressive illness—this may include outpatients with severe or prolonged progressive symptoms or who develop complications such as pneumonia but who are not hospitalized;
3) Any patient who is at higher risk for influenza complications but not hospitalized. Patients in this group include—
- children younger than 2 years (although all children younger than 5 years are considered at higher risk for complications from influenza, the highest risk is for those younger than 2 years)
- adults aged 65 years and older
- persons with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematological (including sickle cell disease), and metabolic disorders (including diabetes mellitus), or neurologic and neurodevelopment conditions (including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy [seizure disorders], stroke, intellectual disability [mental retardation], moderate to severe developmental delay, muscular dystrophy, or spinal cord injury)
- people with immunosuppression, including that caused by medications or by HIV infection
- women who are pregnant or postpartum (within 2 weeks after delivery)
- people aged younger than 19 years who are receiving long-term aspirin therapy
- American Indians/Alaska Natives
- people with extreme obesity (i.e., body-mass index is equal to or greater than 40)
- residents of nursing homes and other chronic-care facilities
3. Timing of Treatment and Implications for Patient Evaluation, Treatment, and Testing
Clinical benefit is greatest when antiviral treatment is administered as early as possible after illness onset. Therefore, antiviral treatment should be started as soon as possible after illness onset and should not be delayed even for a few hours to wait for the results of testing. Ideally, treatment should be initiated within 48 hours of symptom onset. However, antiviral treatment initiated later than 48 hours after illness onset can still be beneficial for some patients.
A very large observational study of more than 29,000 hospitalized influenza patients reported that while the greatest clinical benefit was found when antiviral treatment was initiated within 48 hours of illness onset, starting antiviral treatment more than 2 days after onset had survival benefit in adults versus no treatment. 6 Also, a randomized, placebo-controlled study suggested clinical benefit when oseltamivir was initiated 72 hours after illness onset among febrile children with uncomplicated influenza.7 Clinical judgment, on the basis of the patient’s disease severity and progression, age, underlying medical conditions, likelihood of influenza, and time since onset of symptoms, is important when making antiviral treatment decisions for outpatients, particularly those who are not at increased risk for influenza complications.
Because of the importance of early treatment, decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza. Therefore, empiric antiviral treatment should generally be initiated as soon as possible when there is known influenza activity in the community. A history of current season influenza vaccination does not exclude a diagnosis of influenza in an ill child or adult. During influenza season especially, high-risk patients should be advised to call their provider promptly if they have symptoms of influenza. It may be useful for providers to implement phone triage lines to enable high-risk patients to discuss symptoms over the phone. To facilitate early initiation of treatment, when feasible, an antiviral prescription can be provided without testing and before an office visit.
4. Influenza Testing
Information to assist clinicians about influenza testing decisions is available at https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm. The most accurate influenza tests are molecular assays. Rapid molecular assays are available in clinical settings that can detect influenza virus nucleic acids in respiratory specimens in 15-30 minutes with high sensitivity and specificity. Other approved molecular assays can yield results in 60-80 minutes or in several hours with very high sensitivity and specificity.
For hospitalized patients with suspected influenza, molecular assays are recommended. Information on influenza molecular assays is available at https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm. Rapid influenza diagnostic tests (RIDTs) with an analyzer device can detect influenza A and B viral nucleoprotein antigens in respiratory specimens in 10-15 minutes with moderate sensitivity, and RIDTs without an analyzer device have low to moderate sensitivity compared with reverse transcription-polymerase chain reaction (RT-PCR).
Proper interpretation of influenza testing results is important to guide optimal management of influenza patients. An algorithm to assist clinicians in interpreting the results of influenza testing when influenza viruses ARE circulating in the community is available at https://www.cdc.gov/flu/professionals/diagnosis/algorithm-results-circulating.htm. Clinicians should be aware that a negative RIDT result does not exclude a diagnosis of influenza in a patient with suspected influenza when there is influenza activity in the community. Other factors such as the quality of the specimen, the source of the specimen in the respiratory tract, and the timing of specimen collection in relationship to illness onset, may also affect test results.
5. Antivirals in Non-High Risk Patients with Uncomplicated Influenza
Neuraminidase inhibitors can benefit other individuals with influenza. While current guidance focuses on antiviral treatment of those with severe illness or at high risk of complications from influenza, antiviral treatment may be prescribed on the basis of clinical judgment for any previously healthy (non-high risk) outpatient with suspected or confirmed influenza who presents within 2 days after illness onset. Neuraminidase inhibitors can reduce the duration of uncomplicated influenza illness by approximately 1 day when started within 2 days after illness onset in otherwise healthy persons. It is possible that antiviral treatment started after 48 hours may offer some benefit. 7
6. Antiviral Medications
Three prescription neuraminidase inhibitor antiviral medications are approved by the U.S. Food and Drug
Administration (FDA) and are recommended for use in the U.S. during the 2017–2018 influenza season: oseltamivir (available as a generic version or under the trade name Tamiflu®), zanamivir (Relenza®), and peramivir (Rapivab®).
- Oral oseltamivir is FDA-approved for treatment of uncomplicated influenza within 2 days of illness onset in persons aged 2 weeks and older, and for chemoprophylaxis to prevent influenza in people 1 year of age and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants younger than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year of age, is recommended by CDC and the American Academy of Pediatrics. Due to limited data, use of oseltamivir for chemoprophylaxis is not recommended in children younger than 3 months unless the situation is judged critical. CDC recommends oseltamivir treatment as soon as possible for hospitalized patients with suspected or confirmed influenza, high-risk outpatients with suspected or confirmed influenza, and those with progressive disease.
- Inhaled zanamivir is FDA-approved for treatment of uncomplicated influenza within 2 days of illness onset in persons 7 years and older and for prevention of influenza in persons 5 years and older. Inhaled zanamivir is not recommended for treatment of influenza in hospitalized patients due to limited data.
- Intravenous peramivir is FDA-approved for the treatment of acute uncomplicated influenza within 2 days of illness onset in persons aged 2 years and older.
Adamantanes (rimantadine and amantadine) are not currently recommended for antiviral treatment or chemoprophylaxis of influenza A because of high levels of resistance among circulating influenza A viruses.
There are no current national shortages of neuraminidase inhibitors (i.e., oseltamivir, zanamivir and peramivir), and manufacturers report they expect to meet projected seasonal demands. If there is difficulty locating oseltamivir for oral suspension, as there has been in some previous seasons, oral suspension can be compounded by a pharmacy from oseltamivir capsules. However, this compounded suspension should not be used for convenience or when oseltamivir oral suspension is commercially available.
More information about compounding an oral suspension from oseltamivir 75 mg capsules can be found at https://www.gene.com/download/pdf/tamiflu_prescribing.pdf
Additional Considerations for Clinicians
- Bacterial Infections: Antibiotics are not effective against influenza virus infection, and early diagnosis of influenza can reduce the inappropriate use of antibiotics if bacterial co-infection is not suspected. However, because certain bacterial infections can produce symptoms similar to influenza and bacterial infections can occur as a complication of influenza, bacterial infections should be considered and appropriately treated, if suspected. In addition, because pneumococcal infections are a serious complication of influenza infection, current pneumococcal vaccine recommendations for adults 65 years of age or older, as well as adults and children at increased risk for invasive pneumococcal disease due to chronic underlying medical conditions, should be followed (see http://www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm and http://www.cdc.gov/vaccines/vpd-vac/pneumo/vacc-in-short.htm for further information).
- Adverse Events and Antiviral Use: The most common adverse events associated with oral oseltamivir include a slightly increased risk of nausea and vomiting as compared to placebo, with nausea occurring in 10% of adults with influenza who received oseltamivir and 6% of people who received placebo in controlled clinical trials (3% and 4%, respectively, in children), and vomiting occurring in 9% of adults with influenza who received oseltamivir and 3% of people who received placebo in controlled clinical trials (15% and 9%, respectively, in children). These symptoms are generally transient and can be mitigated if oseltamivir is taken with food. Adverse events for inhaled zanamivir were not increased as compared to placebo in clinical trials, but cases of bronchospasm have been reported during post marketing; inhaled zanamivir is not recommended for persons with underlying airways disease (e.g., asthma or chronic obstructive pulmonary diseases). For people who received peramivir intravenously or intramuscularly in clinical trials, the most common adverse event was diarrhea, occurring in 8% versus 7% in people who received placebo.
Resources for Patient Education
Results from unpublished CDC qualitative research shows that most people interviewed were not aware that drugs to treat influenza illness are available. A fact sheet for patients is available at http://www.cdc.gov/flu/antivirals/whatyoushould.htm.
Note the following important background information for patients:
- If you get the flu, antiviral drugs are a treatment option.
- It is very important that antiviral drugs are used early to treat hospitalized patients, people with severe flu illness, and people who are at high risk for flu complications because of their age, severity of illness, or underlying medical conditions.
- If you have severe illness or are at high risk of serious flu complications, you may be treated with flu antiviral drugs if you get the flu.
- If you have a high-risk condition, treatment with an antiviral drug can mean the difference between having milder illness instead of very serious illness that could result in a hospital stay.
- Other people also may be treated with antiviral drugs by their doctor this season. Most otherwise-healthy people who get the flu, however, do not need to be treated with antiviral drugs.
- Studies show that flu antiviral drugs work best for treatment when they are started within 2 days of getting sick. However, starting antivirals later can still be helpful for some people.
- If your health care provider thinks you have the flu, your health care provider may prescribe antiviral drugs. A test for flu is not necessary.
- Antibiotics are not effective against the flu. Using antibiotics inappropriately can lead to antibiotic resistance and may expose patients to unwanted side effects of the drug.
- Other practices that may help decrease the spread of influenza include respiratory hygiene, cough etiquette, social distancing (e.g., staying home from work and school when ill, staying away from people who are sick) and hand washing.
Additional Resources
- Summary of Influenza Antiviral Treatment Recommendations for Clinicians: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- Clinical Description and Lab Diagnosis of Influenza: http://www.cdc.gov/flu/professionals/diagnosis/index.htm
- Guidance for Clinicians on the Use of RT-PCR and Other Molecular Assays for Diagnosis of Influenza Virus Infection: http://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm
- Interim Guidance for Influenza Outbreak Management in Long-Term Care Facilities: http://www.cdc.gov/flu/professionals/infectioncontrol/ltc-facility-guidance.htm
- Influenza Virus Testing in Investigational Outbreaks in Institutional or Other Closed Settings: https://www.cdc.gov/flu/professionals/diagnosis/guide-virus-diagnostic-tests.htm
- FDA Influenza (Flu) Antiviral Drugs and Related Information (including package inserts): http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm100228.htm
References
2 Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and Safety of Oseltamivir in Children: Systematic Review and Individual Patient Data Meta-analysis of Randomized Controlled Trials. Clin Infect Dis. 2017 Nov 23. doi: 10.1093/cid/cix1040. [Epub ahead of print]
3 Hsu J, Santesso N, Mustafa R, Brozek J, Chen YL, Hopkins JP, Cheung A, Hovhannisyan G, Ivanova L, Flottorp SA, Saeterdal I, Wong AD, Uyeki TM, Akl EA, Alonso-Coello P, Smaill F, Schūnemann HJ. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012 Apr 3;156(7):512-24.
4 Doll MK, Winters N, Boikos C, Kraicer-Melamed H, Gore G, Quach C. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother. 2017 Nov 1;72(11):2990-3007.
5 Venkatesan S, Myles PR, Leonardi-Bee J, Muthuri SG, Al Masri M, Andrews N, Bantar C, Dubnoy-Raz G, Gérardin P, Koay ESC, Loh TP, Memish Z, Miller E, Oliva ME, Rath BA, Schweiger B, Tanq JW, Tran D, Vidmar T, Waight PA, Nguyen-Van-Tam JS. Impact of Outpatient Neuraminidase Inhibitor Treatment in Patients Infected With Influenza A(H1N1)pdm09 at High Risk of Hospitalization: An Individual Participant Data Metaanalysis. Clin Infect Dis. 2017 May 15;64(10):1328-1334.
6 Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Al Khuwaitir TS, Al Mamun A, Anovadiya AP, Azziz-Baumgartner E, Báez C, Bassetti M, Beovic B, Bertisch B, Bonmarin I, Booy R, Borja-Aburto VH, Burgmann H, Cao B, Carratala J, Denholm JT, Dominguez SR, Duarte PA, Dubnov-Raz G, Echavarria M, Fanella S, Gao Z, Gérardin P, Giannella M, Gubbels S, Herberg J, Iglesias AL, Hoger PH, Hu X, Islam QT, Jiménez MF, Kandeel A, Keijzers G, Khalili H, Knight M, Kudo K, Kusznierz G, Kuzman I, Kwan AM, Amine IL, Langenegger E, Lankarani KB, Leo YS, Linko R, Liu P, Madanat F, Mayo-Montero E, McGeer A, Memish Z, Metan G, Mickiene A, Mikić D, Mohn KG, Moradi A, Nymadawa P, Oliva ME, Ozkan M, Parekh D, Paul M, Polack FP, Rath BA, Rodríguez AH, Sarrouf EB, Seale AC, Sertogullarindan B, Siqueira MM, Skręt-Magierło J, Stephan F, Talarek E, Tang JW, To KK, Torres A, Törün SH, Tran D, Uyeki TM, Van Zwol A, Vaudry W, Vidmar T, Yokota RT, Zarogoulidis P; PRIDE Consortium Investigators, Nguyen-Van-Tam JS.. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014 May;2(5):395-404.
7 Fry AM, Goswami D, Nahar K, Sharmin AT, Rahman M, Gubareva L, Azim T, Bresee J, Luby SP, Brooks WA. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014 Feb;14(2):109-18.
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and controlling diseases and injuries; enhances health decisions by providing credible information on critical health issues; and promotes healthy living through strong partnerships with local, national and international organizations.
Department of Health and Human Services
HAN Message Types
- Health Alert: Conveys the highest level of importance; warrants immediate action or attention. Example: HAN00001(https://emergency.cdc.gov/han/han00001.asp)
- Health Advisory: Provides important information for a specific incident or situation; may not require immediate action. Example: HAN00346(https://emergency.cdc.gov/han/han00346.asp)
- Health Update: Provides updated information regarding an incident or situation; unlikely to require immediate action. Example: HAN00342(https://emergency.cdc.gov/han/han00342.asp)
- Info Service: Provides general information that is not necessarily considered to be of an emergent nature. Example: HAN00345(https://emergency.cdc.gov/han/han00345.asp)
###
This message was distributed to state and local health officers, state and local epidemiologists, state and local laboratory directors, public information officers, HAN coordinators, and clinician organizations.
###
NASA: California’s December Inferno
Sunday, December 31st, 2017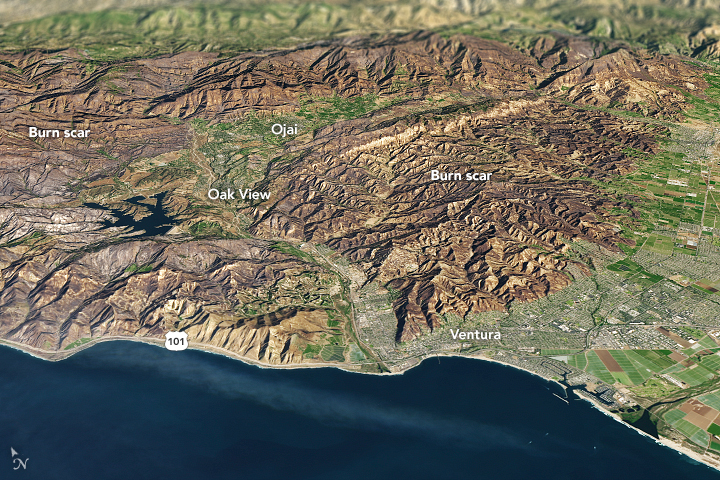
It is rare for large wildfires to burn in California in December, which is usually a wet month for the state. In most years, a few hundreds acres might burn. The 2006 Shekell fire in Ventura charred 13,600 acres, making it the largest December fire in the state between 2000 and 2016.
In 2017, the Thomas fire shattered the record for December and may soon eclipse the worst blaze in any month. After burning for 16 days, the massive fire had scorched 272,000 acres (110,000 hectares or 425 square miles) and was just 60 percent contained. That made it the second largest fire on record in California, trailing only the Cedar fire, which burned 273,246 acres in 2003.
The Operational Land Imager (OLI) on Landsat 8 captured an image of the Thomas fire scar on December 18, 2017. The natural-color Landsat 8 image was draped over an ASTER-derived Global Digital Elevation Model, which shows the topography of the area. The fire raged first near Ventura, then burned the hills around communities of Ojai and Oak View. Firefighters put up a fierce fight and managed to prevent flames from descending into the valley towns. Flames then pushed west toward Summerland, Montecito, and Santa Barbara. As of December 20, the fire was still spreading along the northern edge of the burn scar.
Authorities reported that more than 1,200 structures—most of them in Ventura County—have been destroyed. Several factors came together to make the blaze difficult to control. An usually wet winter and spring in early 2017 caused vegetation to flourish. Then the dry season turned out to be excessively dry, and rains also have been scarce in the typically wetter months of November and December. All of that vegetation dried out and was primed to burn. Once the fire started, warm temperatures and unusually fierce Santa Ana winds caused the fire to spread rapidly.
After nearly two weeks of red flag conditions, a break in the weather has allowed firefighters to beat back the flames in the past few days. But fire officials still do not expect the Thomas fire to be completely contained until January 2018.
-
References
- AccuWeather (2017, December 20) Why the devastating California wildfires have been so unusual, extreme this December. Accessed December 20, 2017.
- InciWeb (2017) Thomas Fire. Accessed December 20, 2017.
- Los Angeles Times (2017, December 30) Another day of reckoning: Return of powerful winds make Thomas fire dangerous again. Accessed December 20, 2017.
- NBC (2017, December 19) Map: How the Thomas Fire Grew Into One of California’s Largest Wildfires. Accessed December 20, 2017.
- The Wrap (2017, December 11) How They Saved Ojai: A Letter From a Town to Its Firefighters. Accessed December 20, 2017.
NASA Earth Observatory image by Joshua Stevens, using Landsat data from the U.S. Geological Survey and ASTER GDEM data from NASA/GSFC/METI/ERSDAC/JAROS, and U.S./Japan ASTER Science Team. Story by Adam Voiland.
- Instrument(s):
- Terra – ASTER
- Landsat 8 – OLI
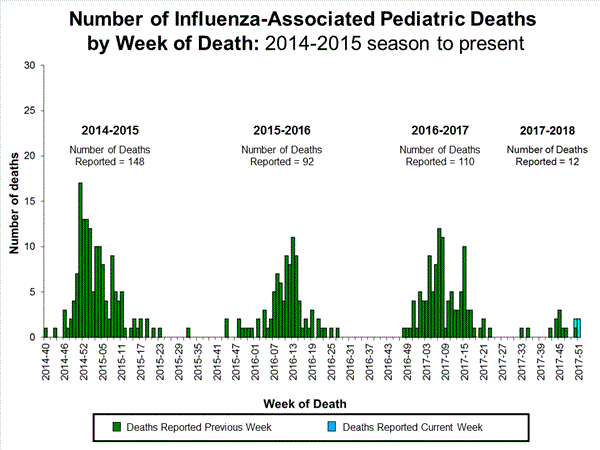
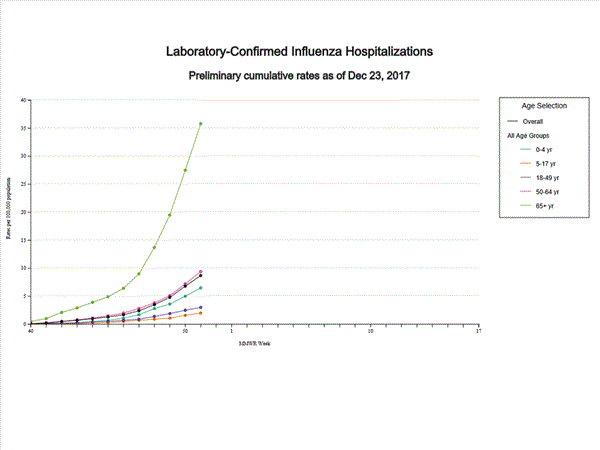
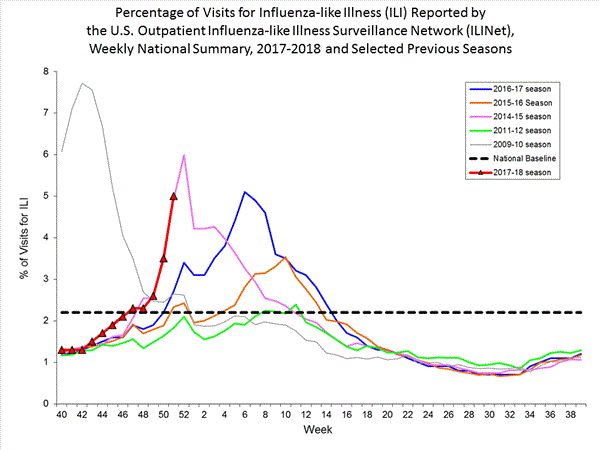
CDC: During week 51 (December 17-23, 2017), influenza activity increased sharply in the United States.
Saturday, December 30th, 2017During week 51 (December 17-23, 2017), influenza activity increased sharply in the United States.
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 51 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
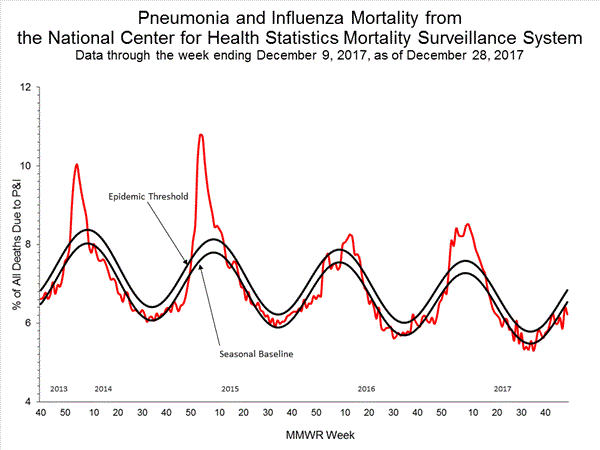
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Three influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 8.7 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 5.0%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. Twenty-one states experienced high ILI activity; New York City and five states experienced moderate ILI activity; eight states experienced low ILI activity; 14 states experienced minimal ILI activity; and the District of Columbia, Puerto Rico and two states had insufficient data.
- Geographic Spread of Influenza:The geographic spread of influenza in 36 states was reported as widespread; Puerto Rico and 13 states reported regional activity; one state reported local activity; and the District of Columbia, the U.S. Virgin Islands, and Guam did not report.
NASA: A Chilly End to 2017 for the Northeast
Saturday, December 30th, 2017
As 2017 drew toward a close, Arctic air spilled into the eastern United States and Canada for several days. Blasts of bitterly cold air set up a white Christmas for many Americans, and forecasters are expecting New Year’s Eve celebrations to be the coldest in recent memory for many areas.
The Moderate Resolution Imaging Spectroradiometer (MODIS) on NASA’s Terra satellite captured this image of the frozen Northeast landscape on December 28, 2017. Brisk northwesterly winds created rows of “cloud streets” as cold air blew over Lake Ontario and the Atlantic Ocean. A layer of snow covered much of New England and upstate New York.
Cloud streets are long parallel bands of cumulus clouds that form when cold air blows over warmer waters and a warmer air layer (temperature inversion) rests over the top of both. The comparatively warm water gives up heat and moisture to the cold air above, and columns of heated air called thermals naturally rise through the atmosphere. The temperature inversion acts like a lid. When the rising thermals hit it, they roll over and loop back on themselves, creating parallel cylinders of rotating air. As this happens, the moisture cools and condenses into flat-bottomed, fluffy-topped cumulus clouds that line up parallel to the direction of the prevailing winds.
While the cold streak has not broken all-time records, it is breaking records for individual days. On the day the image was acquired, weather observers on Mount Washington (New Hampshire) recorded a daily record low of -34 degrees Fahrenheit (-36° Celsius). Baltimore, Boston, Flint, New York, Montreal, and Toronto Cities have seen records fall during this cold snap.
When the ball drops in New York City on New Year’s Eve, forecasters expect air temperatures of 10°F (-12°C), with wind chills of -5°F ( -15°C). The last time it was so cold was in 1962; the record for the coldest ball drop occurred in 1917, when the air temperature was just 1°F (-17°C), according to Jason Samenow of the Capital Weather Gang.
The cold weather pattern has its origins in a large bulge, or ridge, in the jet stream that has brought unseasonably warm weather to Alaska. On the east side of this ridge, a trough in the jet stream plunged southward, bringing plenty of Arctic air with it. This orientation of the jet stream, which looks similar to the greek letter omega, is known as an omega block.
-
References and Further Reading
- Forbes (2017, December 28) A Response For People Using Record Cold U.S. Weather To Refute Climate Change. Accessed December 29, 2017.
- Mashable (2017, December 29) There’s no end in sight to the frigid weather in the U.S. and Canada. Accessed December 29, 2017.
- The Boston Globe (2017, December 28) Mt. Washington summit breaks daily record low. Accessed December 29, 2017.
- The Washington Post (2017, December 29) Eastern U.S. to endure most numbing New Year’s Eve cold in memory. Accessed December 29, 2017.
- The Weather Channel (2017, December 29) Arctic Cold Outbreak to Bring Record Lows to the Plains, Midwest and East Into the New Year. Accessed December 29, 2017.
- Weather Underground (2017, December 25) Almost Half of the Lower 48 States Had Snow Cover Christmas Morning 2017, Including a Rare White Christmas in Seattle. Accessed December 29, 2017.
NASA image by Jeff Schmaltz, LANCE/EOSDIS Rapid Response. Story by Adam Voiland.
- Instrument(s):
- Terra – MODIS
La Niña Makes a Quiet Return
Saturday, December 30th, 2017La Niña is the cooler sister to El Niño. La Niña draws cool water up from the depths of the eastern Pacific, energizes the easterly winds, and pushes warm surface water back toward Asia. (Note the ripples of warm water moving west across the equator.) In turn, global atmospheric circulation and jet streams shift with the changing heat and moisture supply from the vast Pacific Ocean.
During La Niña events, the weather tends to grow warmer and drier across the southern portion of North America, from California to Florida. Cooler than normal temperatures usually prevail across western Canada, Alaska, and the U.S. Pacific Northwest. Over the central and eastern Pacific Ocean, clouds and rainfall become more sporadic, while rainfall tends to increase dramatically in Indonesia and the western Pacific. Weather conditions in late 2017 seemed to fit with that pattern.
Puerto Rico: More than 1.5 million people on the island are still in the dark.
Saturday, December 30th, 2017“….Much of the island’s 2,400 miles off transmission lines, 30,000 miles of distribution lines and 342 substations were damaged in the storm….”
acquired September 27 – 28, 2017
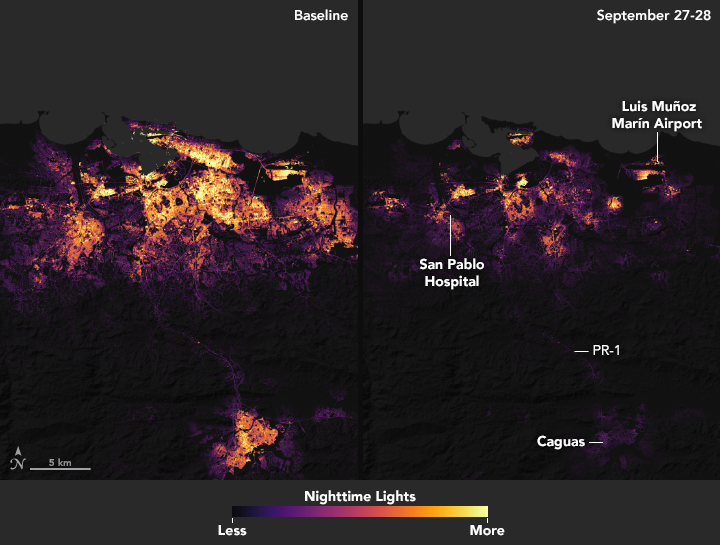
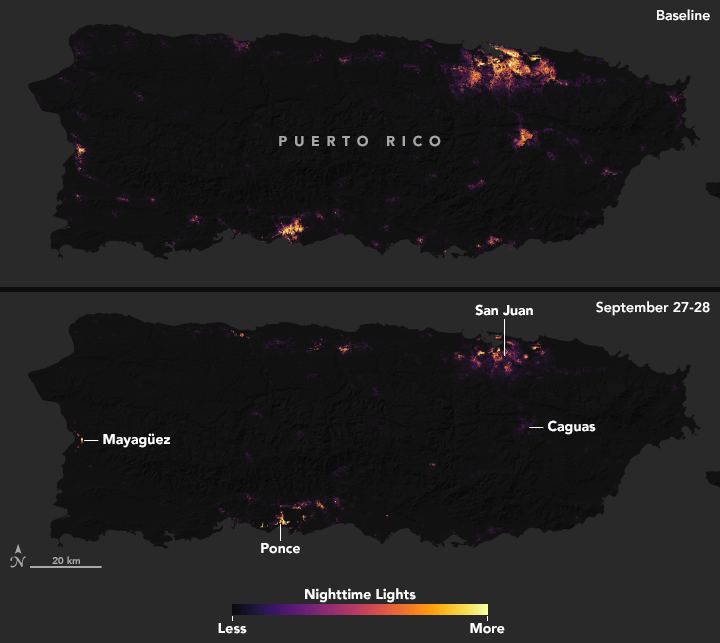
After Hurricane Maria tore across Puerto Rico, it quickly became clear that the destruction would pose daunting challenges for first responders. Most of the electric power grid and telecommunications network was knocked offline. Flooding, downed trees, and toppled power lines made many roads impassable.
In circumstances like this, quickly knowing where the power is out—and how long it has been out—allows first responders to better deploy rescue and repair crews and to distribute life-saving supplies. And that is exactly why teams of scientists at NASA are working long days to make sure that groups like the National Guard and the Federal Emergency Management Agency (FEMA) get high-quality satellite maps of power outages in Puerto Rico.
These before-and-after images of Puerto Rico’s nighttime lights are based on data captured by the Suomi NPP satellite. The data was acquired by the Visible Infrared Imaging Radiometer Suite (VIIRS) “day-night band,” which detects light in a range of wavelengths from green to near-infrared, including reflected moonlight, light from fires and oil wells, lightning, and emissions from cities or other human activity.
The images above show lighting around San Juan, capital of the commonwealth; the images below show the entire island. One image in each pair shows a typical night before Maria made landfall, based upon cloud-free and low moonlight conditions; the second image is a composite that shows light detected by VIIRS on the nights of September 27 and 28, 2017. By compositing two nights, the image has fewer clouds blocking the view. (Note: some clouds still blocked light emissions during the two nights, especially across southeastern and western Puerto Rico.) The images above show widespread outages around San Juan, including key hospital and transportation infrastructure.
At least 41 people were killed and dozens more wounded on Thursday in a bombing at a Shiite cultural center in Kabul, Afghanistan
Friday, December 29th, 2017At least eight people were killed when gunmen opened fire on a Coptic Orthodox Church in Cairo
Friday, December 29th, 2017“……No group has claimed the attack, but experts believe it is the work of the Islamic State.
The militant group declared its intention to attack Egyptian Christians last year as it seeks a stronger foothold in Egypt after defeats in Syria and Iraq. It has since killed more than 100 Egyptian Copts by bombing churches and attacking buses carrying pilgrims…..”
At least 14 died and 16 were injured when a fire engulfed an upscale rooftop restaurant in central Mumbai.
Friday, December 29th, 2017‘…….“There was a stampede and someone pushed me,” said Sulbha Arora, a gynaecologist, in a tweet. “People were running over me even as the ceiling above me was collapsing in flames. Still don’t know how I got out alive.”……..Police filed a complaint against Hitesh Sanghvi, Jigar Sanghvi, and Abhijit Manka, owners of a restaurant in the building, and charged them with causing death due to negligence……’