Archive for the ‘Zika virus’ Category
The World’s Deadliest Animal: Aedes Albopictus Mosquito
Monday, December 16th, 2019The meager, long-legged insect that annoys, bites, and leaves you with an itchy welt is not just a nuisance―it’s one of the world’s most deadly animals. Spreading diseases such as malaria, dengue, West Nile, yellow fever, Zika, chikungunya, and lymphatic filariasis, the mosquito kills more people than any other creature in the world.
In 2018, the number of severe cases of West Nile virus was nearly 25% higher in the Continental U.S. than the average incidence from 2008 to 2017.
In the past 30 years, the worldwide incidence of dengue has risen 30-fold. Forty percent of the world’s population, about 3 billion people, live in areas with a risk of dengue. Dengue is often a leading cause of illness in areas of risk.
Lymphatic filariasis (LF), a parasitic disease transmitted through repeated mosquito bites over a period of months, affects more than 120 million people in 72 countries
In 2017, 435,000 people died from malaria and millions become ill each year including about 2,000 returning travelers in the United States. Nearly half of the world’s population is at risk of this preventable disease.
You can protect yourself from these diseases by avoiding bites from infected mosquitoes.
CDC is committed to providing scientific leadership in fighting these diseases, at home and around the world. From its origins, CDC played a critical role in eliminating malaria from the U.S.

CDC Entomologist, Seth Irish, examines a discarded water bottle for the presence of mosquito larvae, during a training exercise in Dhaka, Bangladesh
Since 2001, global health action has cut the number of malaria deaths in half―saving almost 7 million lives. CDC co-implements the President’s Malaria Initiative in 24 countries and leads Malaria Zero efforts to eliminate malaria from Haiti and efforts to eliminate lymphatic filariasis from Haiti and America Samoa. Haiti is an example of how Mass Drug Administration can reduce spread of LF.
Today, CDC works to eliminate the global burden of malaria and other mosquito-borne diseases. From conducting research to developing tools and approaches to better prevent, detect, and control mosquito-borne diseases, to mitigating drug and insecticide resistance, to accelerating progress towards disease elimination, CDC scientists are working around the world to protect people from mosquito-borne diseases.
2017 Zika Outbreak in Cuba: 1,384 cases reported by Cuban officials that year. Who knew?
Monday, August 26th, 2019“…….Because most cases of Zika go unconfirmed the outbreak actually may have comprised tens of thousands of infections. ……..”
Cell
August 20: World Mosquito Day
Tuesday, August 20th, 2019“Zika has completely fallen off the radar, but the lack of media attention doesn’t mean it’s disappeared,” said Dr. Karin Nielson, a pediatric infectious disease specialist at U.C.L.A.
Wednesday, July 3rd, 2019Document:
Countries and territories with established Aedes aegypti mosquito vectors, but no known cases of Zika virus transmission:
AFRO
Benin; Botswana; Chad; Comoros; Congo; Democratic Republic of the Congo; Equatorial Guinea; Eritrea; Gambia; Ghana; Guinea; Kenya; Liberia; Madagascar; Malawi; Mali; Mauritius; Mayotte; Mozambique; Namibia; Niger; Réunion; Rwanda; Sao Tome and Principe; Seychelles; Sierra Leone; South Africa; South Sudan; Togo; United Republic of Tanzania; Zambia; Zimbabwe
32
AMRO/PAHO Uruguay 1 EMRO Djibouti; Egypt; Oman; Pakistan; Saudi Arabia; Somalia; Sudan; Yemen 8 EURO Georgia; Região Autónoma da Madeira – Portugal; Russian Federation; Turkey 4 SEARO Bhutan; Nepal; Sri Lanka; Timor-Leste 4
WPRO
Australia; Brunei Darussalam; China; Christmas Island; Guam; Kiribati; Nauru; Niue; Northern Mariana Islands (Commonwealth of the); Tokelau; Tuvalu; Wallis and Futuna
12
61
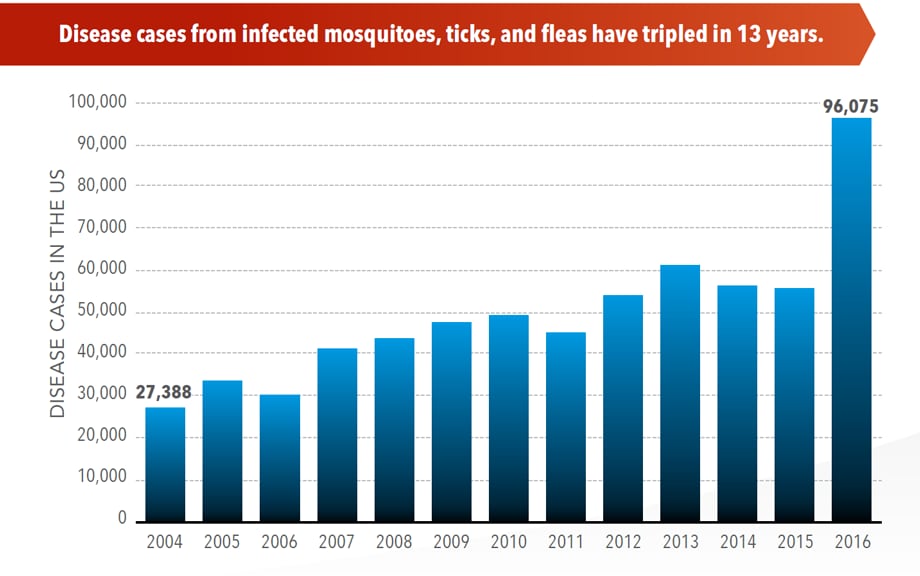
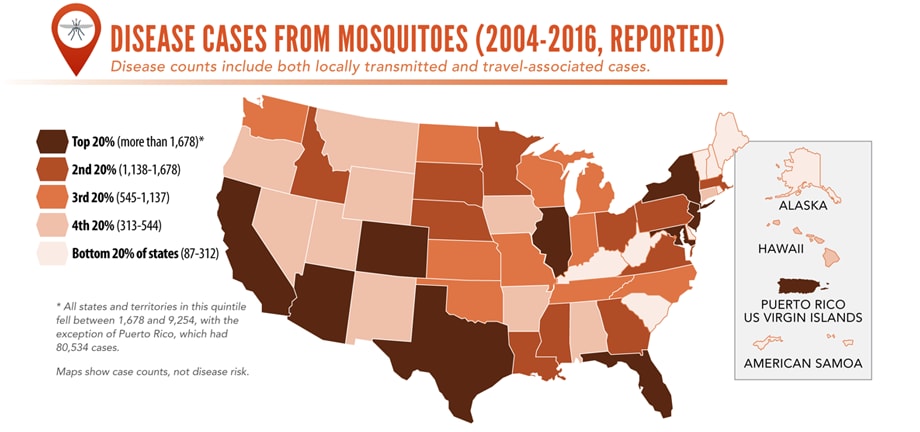
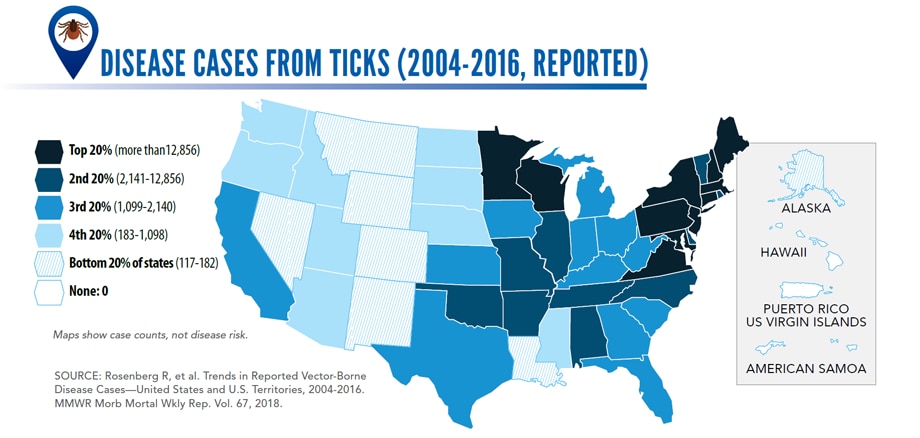
Illnesses on the rise from mosquito, tick, and flea bites
Sunday, June 2nd, 2019Almost everyone has been bitten by a mosquito, tick, or flea. These can be vectors for spreading pathogens (germs). A person who gets bitten by a vector and gets sick has a vector-borne disease, like dengue, Zika, Lyme, or plague. Between 2004 and 2016, more than 640,000 cases of these diseases were reported, and 9 new germs spread by bites from infected mosquitoes and ticks were discovered or introduced in the US. State and local health departments and vector control organizations are the nation’s main defense against this increasing threat. Yet, 84% of local vector control organizations lack at least 1 of 5 core vector control competencies. Better control of mosquitoes and ticks is needed to protect people from these costly and deadly diseases.
State and local public health agencies can
- Build and sustain public health programs that test and track germs and the mosquitoes and ticks that spread them.
- Train vector control staff on 5 core competencies for conducting prevention and control activities. http://bit.ly/2FG1OMwExternal
- Educate the public about how to prevent bites and control germs spread by mosquitoes, ticks, and fleas in their communities.
Increasing threat, limited capacity to respond
More cases in the US (2004-2016)
- The number of reported cases of disease from mosquito, tick, and flea bites has more than tripled.
- More than 640,000 cases of these diseases were reported from 2004 to 2016.
- Disease cases from ticks have doubled.
- Mosquito-borne disease epidemics happen more frequently.
More germs (2004-2016)
- Chikungunya and Zika viruses caused outbreaks in the US for the first time.
- Seven new tickborne germs can infect people in the US.
More people at risk
- Commerce moves mosquitoes, ticks, and fleas around the world.
- Infected travelers can introduce and spread germs across the world.
- Mosquitoes and ticks move germs into new areas of the US, causing more people to be at risk.
The US is not fully prepared
-
- Local and state health departments and vector control organizations face increasing demands to respond to these threats.
- More than 80% of vector control organizations report needing improvement in 1 or more of 5 core competencies, such as testing for pesticide resistance.
- More proven and publicly accepted mosquito and tick control methods are needed to prevent and control these diseases.
“Scary Monsters and Nice Sprites” in the fight against Aedes Aegyptii
Friday, April 5th, 2019“…..The observation that such music can delay host attack, reduce blood feeding, and disrupt mating provides new avenues for the development of music-based personal protective and control measures against Aedes-borne diseases…..”
Acta Tropica
Volume 194, June 2019, Pages 93-99

The electronic song “Scary Monsters and Nice Sprites” reduces host attack and mating success in the dengue vector Aedes aegypti
Latest research: Immune globulin shows promise for severe Zika thrombocytopenia
Monday, December 10th, 2018Elizabeth A Van Dyne, Paige Neaterour, Aidsa Rivera, Melissa Bello-Pagan, Laura Adams, Jorge Munoz-Jordan, Priscilla Baez, Myriam Garcia, Stephen H Waterman, Nimia Reyes, Lisa C Richardson, Brenda Rivera-Garcia, Tyler M Sharp; Incidence and Outcome of Severe and Non-severe Thrombocytopenia Associated with Zika Virus Infection — Puerto Rico, 2016, Open Forum Infectious Diseases, , ofy325, https://doi.org/10.1093/ofid/ofy325
“…..Of 37,878 patients with ZIKV infection, 47 (0.1%) had thrombocytopenia in the absence of an alternative etiology (1.4 cases/100,000 population), including 12 with severe thrombocytopenia. Most patients with thrombocytopenia were adult (77%) and male (53%). Platelet nadir occurred a median of six (range: 1–16) and five (range: 0–34) days after symptom onset for patients with severe and non-severe thrombocytopenia, respectively. Among patients with severe thrombocytopenia, all had bleeding, 33% were admitted to the ICU, and 8% died; 50% were treated for ITP. Among five patients with severe thrombocytopenia who received intravenous immunoglobulin, median platelet count increase was 112 X 10 9/L (range: 65–202 X 10 9/L). In contrast, among four patients who received platelet transfusion, median increase in platelet count was 8.5 X 10 9/L (range: -6–52 x 10 9/L)……”
Current Emergency Use Authorizations
Thursday, December 6th, 2018
The Emergency Use Authorization (EUA) authority allows FDA to help strengthen the nation’s public health protections against CBRN threats by facilitating the availability and use of MCMs needed during public health emergencies.
Under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), the FDA Commissioner may allow unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when there are no adequate, approved, and available alternatives.
Section 564 of the FD&C Act was amended by the Project Bioshield Act of 2004 and the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), which was enacted in March 2013
Current EUAs
The tables below provide information on current EUAs:
- Anthrax: Doxycycline Mass Dispensing EUA Information and National Postal Model Anthrax EUA Information
- Ebola Virus EUA Information
- Enterovirus D68 (EV-D68) EUA Information
- French Freeze Dried Plasma Information
- H7N9 Influenza EUA Information
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV) EUA Information
- Nerve Agent EUA Information
- Zika Virus EUA Information
India is currently experiencing its largest Zika virus outbreak
Wednesday, November 28th, 2018Alexander G Watts, M S A Carmen Huber, Isaac I Bogoch, Oliver J Brady, Moritz U G Kraemer, Kamran Khan; Potential Zika virus spread within and beyond India, Journal of Travel Medicine, , tay132, https://doi.org/10.1093/jtm/tay132
“……By the end of October, 147 cases had been reported in Jaipur, a popular tourist destination, and as of Nov 2, neighboring Gujarat state reported 1 case and Madhya Pradesh state reported 3 infections…..”