Archive for the ‘Plague’ Category
The Third Plague Pandemic
Wednesday, June 5th, 2019The Third Plague Pandemic in Europe
“……The Third Plague Pandemic originated in the Yunnan region of southwest China, where plague caused multiple outbreaks since 1772 [15–17]. In 1894, plague reached Canton and then spread to Hong Kong, where Alexandre Yersin identified the bacterium.It was then carried by ships to Japan, Singapore, Taiwan and the Indian subcontinent [18,19]. Over the next few years, plague spread to many cities around the world: Bombay, Singapore, Alexandria, Buenos Aires, Rio de Janeiro, Honolulu, San Francisco and Sidney, among others [20]. The earliest known European cases occurred in September and October 1896, when two sailors from Bombay died of plague on ships docked in London on the Thames [21]…….There were 1692 cases and 457 deaths from plague reported in Europe between 1899 and 1947 (figure 1; electronic supplementary material, table S1), with the largest number of cases in the years 1899 and 1920. Cases were geographically widespread, although they were primarily found in coastal or inland port cities (figure 2). Plague was reported in 11 countries, and many cities, including Lisbon, Marseille, Paris and Pireas, experienced multiple outbreaks…….”
Illnesses on the rise from mosquito, tick, and flea bites
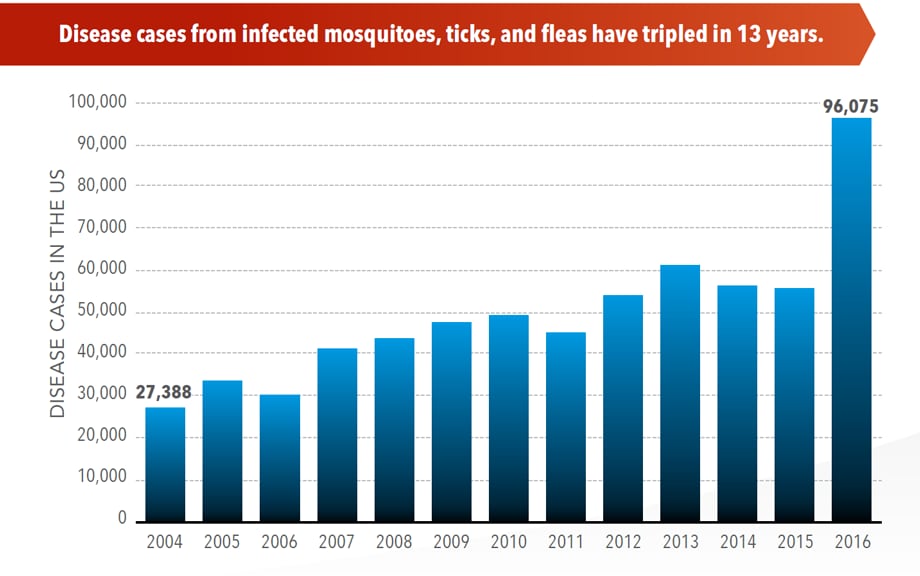
Sunday, June 2nd, 2019Almost everyone has been bitten by a mosquito, tick, or flea. These can be vectors for spreading pathogens (germs). A person who gets bitten by a vector and gets sick has a vector-borne disease, like dengue, Zika, Lyme, or plague. Between 2004 and 2016, more than 640,000 cases of these diseases were reported, and 9 new germs spread by bites from infected mosquitoes and ticks were discovered or introduced in the US. State and local health departments and vector control organizations are the nation’s main defense against this increasing threat. Yet, 84% of local vector control organizations lack at least 1 of 5 core vector control competencies. Better control of mosquitoes and ticks is needed to protect people from these costly and deadly diseases.
State and local public health agencies can
- Build and sustain public health programs that test and track germs and the mosquitoes and ticks that spread them.
- Train vector control staff on 5 core competencies for conducting prevention and control activities. http://bit.ly/2FG1OMwExternal
- Educate the public about how to prevent bites and control germs spread by mosquitoes, ticks, and fleas in their communities.
Increasing threat, limited capacity to respond
More cases in the US (2004-2016)
- The number of reported cases of disease from mosquito, tick, and flea bites has more than tripled.
- More than 640,000 cases of these diseases were reported from 2004 to 2016.
- Disease cases from ticks have doubled.
- Mosquito-borne disease epidemics happen more frequently.
More germs (2004-2016)
- Chikungunya and Zika viruses caused outbreaks in the US for the first time.
- Seven new tickborne germs can infect people in the US.
More people at risk
- Commerce moves mosquitoes, ticks, and fleas around the world.
- Infected travelers can introduce and spread germs across the world.
- Mosquitoes and ticks move germs into new areas of the US, causing more people to be at risk.
The US is not fully prepared
-
- Local and state health departments and vector control organizations face increasing demands to respond to these threats.
- More than 80% of vector control organizations report needing improvement in 1 or more of 5 core competencies, such as testing for pesticide resistance.
- More proven and publicly accepted mosquito and tick control methods are needed to prevent and control these diseases.
Weekly summary of major outbreaks in Africa
Wednesday, March 13th, 2019Plague: Uganda
2 Cases
1 Death: 50% CFR
Ebola virus disease: Democratic Republic of the Congo
921 Cases
582 Deaths: 63% CFR
Hepatitis E: Namibia
4 669 Cases
41 Deaths: 0.9% CFR
Lassa fever: Nigeria
420 Cases
93 Deaths: CFR 22.1%
A Dual Vaccine against Anthrax and Plague
Wednesday, December 5th, 2018WHO: Prioritizing Emerging Infectious Diseases in Need of Research and Development
Sunday, August 19th, 2018The World Health Organization R&D Blueprint aims to accelerate the availability of medical technologies during epidemics by focusing on a list of prioritized emerging diseases for which medical countermeasures are insufficient or nonexistent. The prioritization process has 3 components: a Delphi process to narrow down a list of potential priority diseases, a multicriteria decision analysis to rank the short list of diseases, and a final Delphi round to arrive at a final list of 10 diseases.
A group of international experts applied this process in January 2017, resulting in a list of 10 priority diseases. The robustness of the list was tested by performing a sensitivity analysis. The new process corrected major shortcomings in the pre–R&D Blueprint approach to disease prioritization and increased confidence in the results.
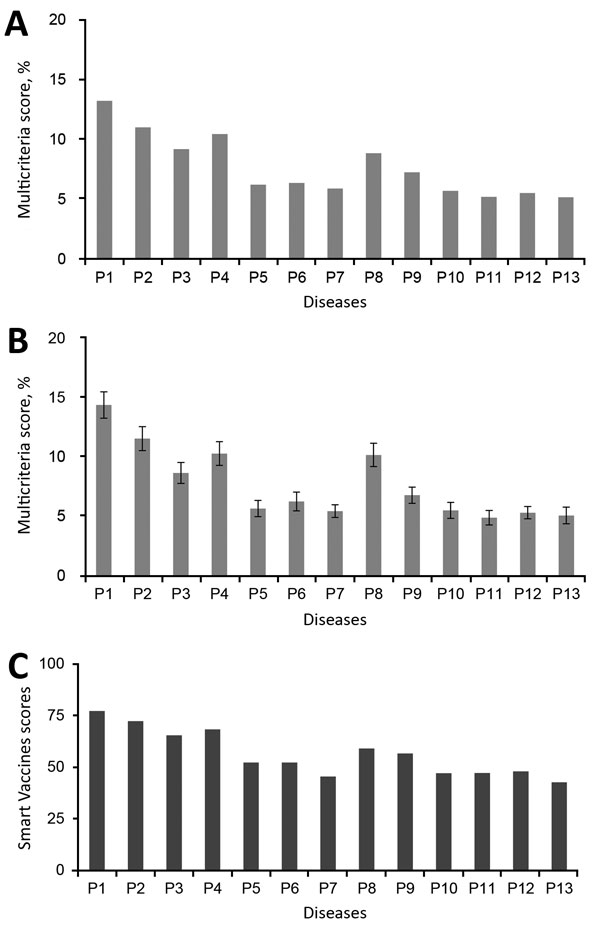
Multicriteria scores of diseases considered in the 2017 prioritization exercise for the development of the World Health Organization R&D Blueprint to prioritize emerging infectious diseases in need of research and development. A) Disease final ranking using the geometric average of the comparison matrices. B) Disease final ranking using the arithmetic average of the raw data. Error bars correspond to SD, indicating disagreement among experts. C) Disease final ranking using the SMART Vaccines prioritization tool (56). P1, Ebola virus infection; P2, Marburg virus infection; P3, Middle East Respiratory Syndrome coronavirus infection; P4, severe acute respiratory syndrome; P5, Lassa virus infection; P6, Nipah virus infection; P7, Rift Valley fever; P8, Zika virus infection; P9, Crimean-Congo hemorrhagic fever; P10, severe fever with thrombocytopenia syndrome; P11, South American hemorrhagic fever; P12, plague; P13, hantavirus infection.
Si Mehand M, Millett P, Al-Shorbaji F, Roth C, Kieny MP, Murgue B. World Health Organization methodology to prioritize emerging infectious diseases in need of research and development. Emerg Infect Dis. 2018 Sep [date cited]. https://doi.org/10.3201/eid2409.171427
Peru: Un hombre de 42 años, murió a consecuencia de la peste bubónica
Monday, July 16th, 2018“…..the patient, who
had been living in the USA for 7 years, was admitted to the Regional
Hospital of Lambayeque, where doctors confirmed bubonic plague, which
later developed into septicemic plague. The specialist said that the
citizen had high fever and malaise…..”

The number of reported cases of disease from mosquito, tick, and flea bites has more than tripled in the USA (2004-2016)
Wednesday, May 2nd, 2018More cases in the US (2004-2016)
- The number of reported cases of disease from mosquito, tick, and flea bites has more than tripled.
- More than 640,000 cases of these diseases were reported from 2004 to 2016.
- Disease cases from ticks have doubled.
- Mosquito-borne disease epidemics happen more frequently.
More germs (2004-2016)
- Chikungunya and Zika viruses caused outbreaks in the US for the first time.
- Seven new tickborne germs can infect people in the US.
More people at risk
- Commerce moves mosquitoes, ticks, and fleas around the world.
- Infected travelers can introduce and spread germs across the world.
- Mosquitoes and ticks move germs into new areas of the US, causing more people to be at risk.
The US is not fully prepared
- Local and state health departments and vector control organizations face increasing demands to respond to these threats.
- More than 80% of vector control organizations report needing improvement in 1 or more of 5 core competencies, such as testing for pesticide resistance.
- More proven and publicly accepted mosquito and tick control methods are needed to prevent and control these diseases.
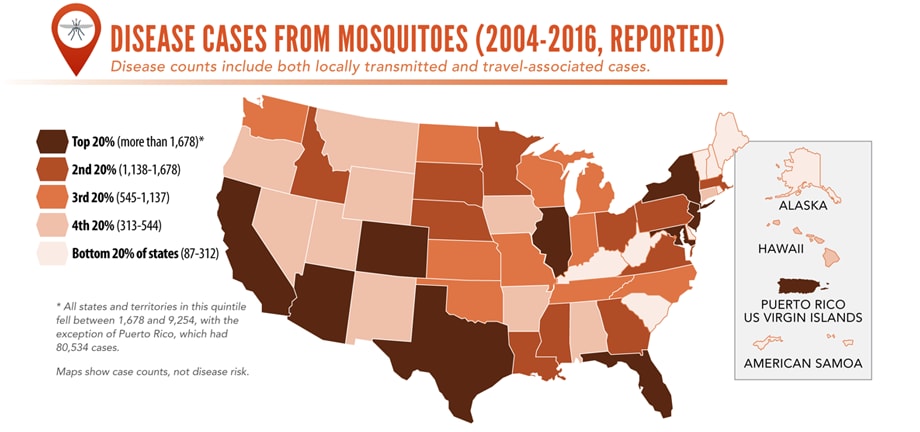
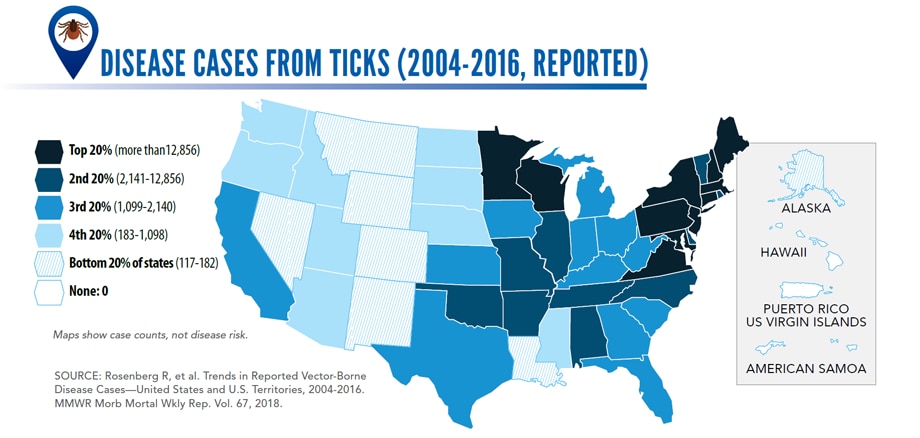
Vector-Borne Diseases Reported by States to CDC

Mosquito-borne diseases
- California serogroup viruses
- Chikungunya virus
- Dengue viruses
- Eastern equine encephalitis virus
- Malaria plasmodium
- St. Louis encephalitis virus
- West Nile virus
- Yellow fever virus
- Zika virus

Tickborne diseases
- Anaplasmosis/ehrlichiosis
- Babesiosis
- Lyme disease
- Powassan virus
- Spotted fever rickettsiosis
- Tularemia
Fleaborne disease
- Plague
For more information: https://wwwn.cdc.gov/nndss/
Human ectoparasites [(i.e. human fleas (Pulex irritans) or body lice (Pediculus humanus humanus)] were primary vectors for plague during the Second Pandemic, including the Black Death (1346–1353)
Wednesday, January 17th, 2018Human ectoparasites and the spread of plague in Europe during the Second Pandemic
PNAS 2018 : 1715640115v1-201715640.
Pneumonic Plague in Johannesburg, South Africa, 1904
Thursday, December 21st, 2017Volume 24, Number 1—January 2018
Historical Review
Evans CM, Egan JR, Hall I. Pneumonic Plague in Johannesburg, South Africa, 1904. Emerg Infect Dis. 2018;24(1):95-102. https://dx.doi.org/10.3201/eid2401.161817
Pneumonic Plague in Johannesburg, South Africa, 1904
Figure 3
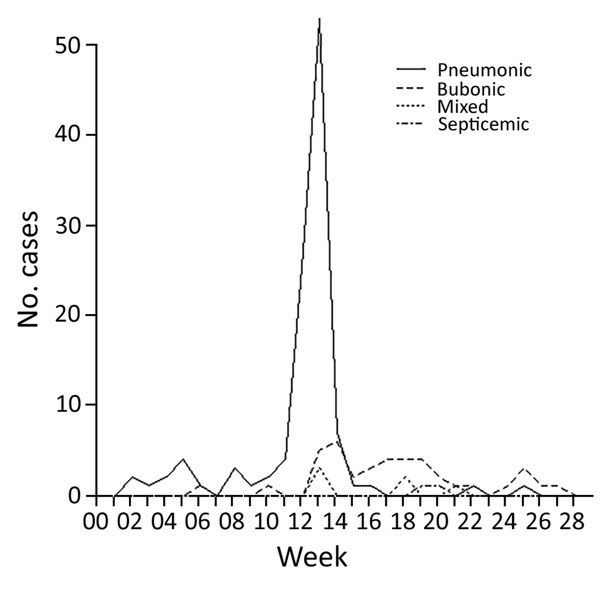
Figure 3. Incidence of the 4 types of plague over the duration of the epidemic in Johannesburg, South Africa, from week ending January 2 to week ending June 16, 1904.
Figure 4

Figure 4. A) Deaths per day resulting from primary pneumonic plague in Johannesburg, South Africa, March 7–31, 1904. B) Back-calculated number of case-patients experiencing symptom onset. Circles represent most likely values; error bars represent 95% CIs. C) Transmissibility of primary pneumonic plague as measured by reproduction number, Rt. Circles represent the most likely values, error bars represent 95% CIs, and shaded polygons represent the period over which Rt was estimated. Uncertainty in the back-calculated incidence has not been accounted for in the transmission estimates, which means that the variations in the time-varying Rt are probably underestimated because the incidence curve is smoothed out somewhat by the back-calculation process (and also reduced slightly because of rounding to the nearest integer). However, because the 7-day sliding window has the effect of smoothing out the Rt estimates in any case, not accounting for the uncertainty in the back-calculation probably has a limited effect on panel C results.
Etymologia: Plague
Wednesday, December 20th, 2017Henry R. Etymologia: Plague. Emerg Infect Dis. 2018;24(1):102. https://dx.doi.org/10.3201/eid2401.ET2401
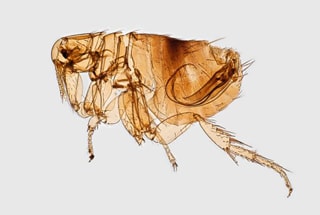
Plague (from the Latin plaga, “stroke” or “wound”) infections are believed to have been common since at least 3000 bce. Plague is caused by the ancestor of current Yersinia (named for Swiss bacteriologist Alexandre Yersin, who first isolated the bacterium) pestis strains (Figure 1). However, this ancestral Y. pestis lacked the critical Yersinia murine toxin (ymt) gene that enables vectorborne transmission. After acquiring this gene (sometime during 1600–950 bce), which encodes a phospholipase D that protects the bacterium inside the flea gut, Y. pestis evolved the ability to cause pandemics of bubonic plague. The first recoded of these, the Justinian Plague, began in 541 ace and eventually killed more than 25 million persons (Figure 2)
Figure 1
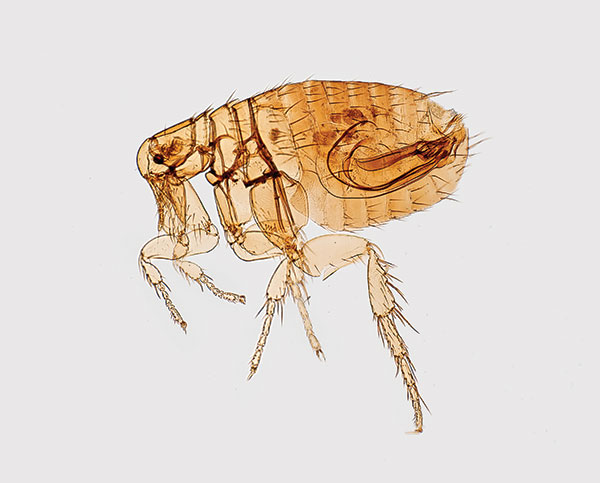
Figure 1. Digitally colorized scanning electron microscopic image of a flea. Fleas are known to carry a number of diseases that are transferable to humans through their bites, including plague, caused by the bacterium Yersinia pestis. Photo: Centers for Disease Control and Prevention (CDC), Janice Haney Carr.
Figure 2

Figure 2. Plague warning signs posted in regions where plague has been discovered. In remote areas with little human habitation, the most appropriate action may be to post signs on the roads entering the epizootic area to warn people, and provide information on personal protection and plague prevention. Photo, CDC, 1993.