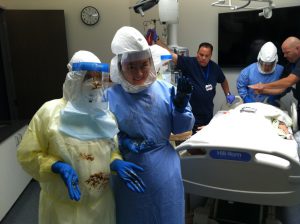
CDC Director Robert Redfield,the Minister of State for Health Sarah Opendi, and the US Ambassador to Uganda, Deborah Malac learn about Uganda Preparedness activities in 2018. Photo credit: Irene Nabusoba
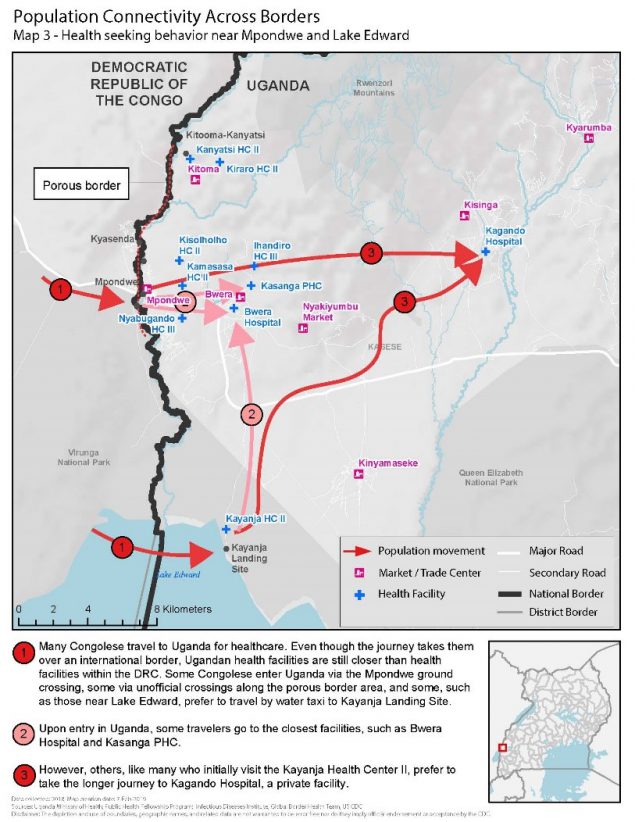
On April 11, 2019, the Uganda MOH, CDC, and other partners held a nationwide Ebola outbreak simulation exercise. The three scenarios were: arrival of an ill traveler at Entebbe International Airport, detection of an ill person referred to Kagando Hospital in Kasese District, and identification of an ill traveler at a border crossing in Kasese. All three scenarios involved the PHEOC in the capital, Kampala.
Stopping Ebola requires early identification of cases, effective isolation of people ill with Ebola, and contact tracing of people exposed to Ebola patients so they can be vaccinated and isolated if they develop symptoms. The exercise evaluated the operational capabilities and identified strengths, weaknesses, and opportunities for improvement of Uganda’s emergency management systems at the border, in communities, health facilities, districts, and at the national level. Key capacities assessed included: Ebola alert management systems at key points of entry; isolation, transportation, and management of people with a suspected case of Ebola; laboratory sample collection, packaging, and dispatch; and initial management of people confirmed to have Ebola. The exercise also looked at community engagement in case of an Ebola event, knowledge and skills of village health teams, capacity of health facilities to handle cases, and general logistics and coordination at the district and national levels.
“Our aim with the simulation was to build full capabilities for full-fledged, real-time deployment and response. This made a world of a difference when Ebola crossed the border,” said CDC Uganda Division of Global Health Protection program director, Jaco Homsy.