Archive for March, 2018
The Greek island of Lesbos: Migration crisis
Saturday, March 31st, 2018“…..Some 5,500 people are detained in Moria, about 2,500 more than the camp was designed to hold……..
Rain soaks through the tents, and there is a lack of electricity and hot water in the showers, even in winter. The public toilets and showers are soiled with feces. As bad as the food is, it often runs out. The lines — for everything — are endless. Fights break out constantly. Violence, theft and rape are constant threats……”
https://www.youtube.com/watch?v=McRM-Yq78Io
https://www.youtube.com/watch?v=D7qnP9Lvz14
Candida auris: From 2013 through 2017 European officials recorded 620 cases, mostly from four large outbreaks, and 110 of them (17.7%) involved bloodstream infections
Saturday, March 31st, 2018C. auris “is an emerging fungus that is causing difficult-to-control outbreaks of invasive healthcare-associated infections. Since the first report of C. auris in 2009 [2], cases have been reported worldwide. Identification of C. auris requires specialised laboratory methodology as traditional identification methods may lead to misidentification [3,4]. In addition, C. auris has been associated with resistance to multiple antifungal classes [5] and difficulties related to the interpretation of antifungal susceptibility results [6]. The combination of these characteristics, i.e. propensity to cause nosocomial outbreaks, multi-drug resistance, ability to cause severe disease and difficulties with laboratory detection, render C. auris a public health threat ….”
Despite South Sudan’s crippling civil war, the country has interrupted the transmission of Guinea worm disease
Saturday, March 31st, 2018“…..A breakthrough came in 2001 when distribution of over 9 million portable, straw-like plastic pipes with cloth or metal filters allowed each at-risk person access to water free from water fleas. Since then, targeted health education and village volunteers have also contributed…..”
“A not-for-profit, nongovernmental organization, The Carter Center has helped to improve life for people in over 80 countries by resolving conflicts; advancing democracy, human rights, and economic opportunity; preventing diseases; and improving mental health care. The Carter Center was founded in 1982 by former U.S. President Jimmy Carter and former First Lady Rosalynn Carter, in partnership with Emory University, to advance peace and health worldwide.”

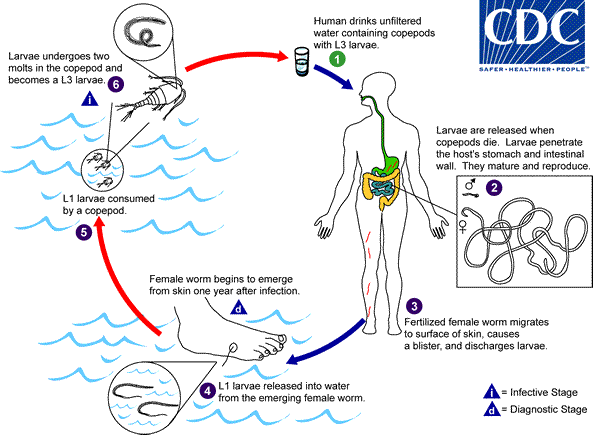
Humans become infected by drinking unfiltered water containing copepods (small crustaceans) which are infected with larvae of D. medinensis ![]() . Following ingestion, the copepods die and release the larvae, which penetrate the host stomach and intestinal wall and enter the abdominal cavity and retroperitoneal space
. Following ingestion, the copepods die and release the larvae, which penetrate the host stomach and intestinal wall and enter the abdominal cavity and retroperitoneal space ![]() . After maturation into adults and copulation, the male worms die and the females (length: 70 to 120 cm) migrate in the subcutaneous tissues towards the skin surface
. After maturation into adults and copulation, the male worms die and the females (length: 70 to 120 cm) migrate in the subcutaneous tissues towards the skin surface ![]() . Approximately one year after infection, the female worm induces a blister on the skin, generally on the distal lower extremity, which ruptures. When this lesion comes into contact with water, a contact that the patient seeks to relieve the local discomfort, the female worm emerges and releases larvae
. Approximately one year after infection, the female worm induces a blister on the skin, generally on the distal lower extremity, which ruptures. When this lesion comes into contact with water, a contact that the patient seeks to relieve the local discomfort, the female worm emerges and releases larvae ![]() . The larvae are ingested by a copepod
. The larvae are ingested by a copepod ![]() and after two weeks (and two molts) have developed into infective larvae
and after two weeks (and two molts) have developed into infective larvae ![]() . Ingestion of the copepods closes the cycle
. Ingestion of the copepods closes the cycle ![]() .
.
The Listeriosis outbreak in South Africa is now the largest ever reported to the WHO.
Friday, March 30th, 2018In South Africa, an outbreak of listeriosis, a serious foodborne disease, has been ongoing since the start of 2017. Between 1 January 2017 through 14 March 2018, 978 laboratory-confirmed listeriosis cases have been reported to the National Institute for Communicable Diseases (NICD) from all provinces. The majority of cases have come from three provinces: 581 (59%) from Gauteng, 118 (12%) from Western Cape and 70 (7%) from KwaZulu-Natal provinces, with the remaining cases coming from the other provinces in South Africa. The outcome of illness is known for 674 patients, of whom 183 (27%) of them died; this case fatality rate is comparable to other recorded listeriosis outbreaks worldwide. Most of the cases are persons who have higher risks for a severe disease outcome, such as neonates, pregnant women, the elderly and immunocompromised persons. In this outbreak, 42% of the cases are neonates who were infected during pregnancy or delivery.
Whole genome sequencing was performed on isolates from a large subset of patients. Ninety one percent of the strains belonged to Listeria monocytogenes Sequence Type 6 (ST6). The same ST6 sequence type was identified in a widely consumed ready-to-eat processed meat product called “Polony”. The same strain was also found in the processing environment of the manufacturer of the implicated product. On 4 March 2018, the Ministry of Health, announced that this product was believed to be the source of the outbreak.
The food processing company and three of its retailers export to 15 countries1 in the African region . All of these countries have issued recalls for the implicated products. Environmental samples from other food production companies in South Africa have also tested positive for Listeria but with strains different from the outbreak strain. These companies have also issued food recalls. Some of their products have been exported to some of the countries mentioned above.
Nine percent of the reported cases in the present outbreak in South Africa were infected with different strains of Listeria than the predominant ST6 outbreak strain. This may indicate that more than one outbreak is ongoing. Comprehensive investigations to identify the source of infection of these cases should be conducted.
Number of Laboratory-Confirmed Cases of Listeriosis by Week of Sample Collection and Province, South Africa, 01 January 2017 to 12 March 2018 (n=978)
Source: National Institute for Communicable Diseases (NICD), South Africa2.
Public health response
The country has activated a national multisectoral task force to coordinate investigation and response activities:
- The Minister of Health, South Africa, held a press conference on 4 March 2018 to announce the source of the outbreak.
- Following the source identification, the national authorities have taken measures to limit further infections and associated mortality, including but not limited to the issuance of safety recall notices, compliance notices, and measures related to exportation of implicated products and risk communication with vulnerable groups.
- The Southern African Development Community (SADC) Health Ministers held a meeting in Johannesburg on 15 March 2018, to share information and to enhance preparedness and response for listeriosis. Health Ministers were further reminded about their rights and obligations under the International Health Regulations (IHR) with regards to additional health measures for international travel and trade.
- Listeriosis has been made a notifiable medical condition in South Africa Since December 2017.
- National risk communication activities have been initiated since December 2017 around the “WHO five keys to safer food messages”.
WHO risk assessment
Globally, this is the largest outbreak of listeriosis that has been detected. Due to the potentially long incubation period of listeriosis (one to three weeks and up to 70 days) further cases are expected before the impact of the food recall is observed.
Following the identification of the source of this outbreak, WHO is now concerned that the export of implicated products may have resulted in listeriosis cases in other countries. Detailed information on the implicated product batches exported to each of the identified 15 countries should be shared with WHO and the International Food Safety Authorities Network (INFOSAN) Secretariat. This will help to facilitate efficient recalls of these products.
Recently, Namibia reported a confirmed case of listeriosis. It is important that this case and other possible cases be properly investigated and the implicated food sources identified. The isolated Listeria strains should be sequenced to determine potential links to the outbreak in South Africa. Some of these countries may not have well-established surveillance systems and laboratory diagnostic services in place to detect cases of listeriosis. In such cases, WHO is ready to provide support and has reached out to 16 African nations (two West African countries and 14 members of SADC) to provide support for preparedness and response.
An increase in public concern about the outbreak has been reflected in media reports and discussions in various social media platforms.
WHO advice
Countries should strengthen national food safety and disease surveillance systems as a prerequisite to prevent similar events in the future and to ensure a safe food supply for their populations. Additionally, countries are urged to pay closer attention to common foodborne pathogens such as Salmonella species, Campylobacter jejuni, Escherichia coli and Listeria monocytogenes, to make listeriosis a notifiable disease if not yet done, to make the best use of a new “WHO manual to strengthen surveillance of and response to foodborne diseases”, which was published on November 2017 and to refer to the “WHO Factsheet on Listeriosis”. See below, in the “Further Information” section for links to these documents.
Responsible authorities are encouraged to proactively provide public health advice on the prevention and control of listeriosis, including, strengthened risk communication, to address public concerns and promote outbreak control measures .
WHO does not currently recommend any travel or trade restrictions in relation to this outbreak, other than the recall of processed meat products indicated by the South African Government.
While WHO understands the desire by sovereign governments to take measures to protect the health of their populations, States Parties to the IHR have obligations not to take actions that significantly interfere with international travel and trade which are not based on scientific principles and which may be viewed as excessive. Indeed such measures contravene the spirit and purpose of the IHR, and can impede public health objectives.
Currently, 12 out of 15 countries have recalled the implicated processed meat products, and banned imports of the same, while three out of these countries have additionally banned imports of other food products. WHO continues to monitor the travel and trade measures taken by countries in relation to this outbreak and their compliance with requirements under the IHR.
For travellers, it is advisable to practice good food hygiene, such as avoiding uncooked food, avoiding food that has been kept at room temperature for several hours, and always washing hands thoroughly with soap and water before preparing or consuming food.
1The 15 countries include: Angola, Botswana, Democratic Republic of the Congo, Ghana, Lesotho, Madagascar, Malawi, Mauritius, Mozambique, Namibia, Nigeria, Swaziland, Uganda, Zambia, and Zimbabwe.
2The provinces are: Western Cape (WC), North West (NW), Northern Cape (NC), Mpumalanga (MP), Limpopo (LP), KwaZulu-Natal (KZ), Gauteng (GA), Free State (FS) and Eastern Cape (EC).
Further information:
The Brazilian Ministry of Health yesterday noted 211 newly confirmed yellow fever cases, including 38 more deaths.
Friday, March 30th, 2018“O Brasil confirmou 1.131 casos e 338 óbitos no período de 1º julho de 2017 a 27 de março deste ano. No mesmo período do ano passado, foram confirmados 660 casos e 210 óbitos……”
Medical countermeasures (MCMs) to treat patients with radiation-induced myelosuppression following a radiological/nuclear incident
Friday, March 30th, 2018MCMs to treat patients with radiation-induced myelosuppression following a radiological/nuclear incident (H-ARS)
Myelosuppression occurs when radiation damages the bone marrow. Suppression of the bone marrow blocks the production of blood cells. There are FDA-approved products that can help patients with H-ARS by facilitating recovery of bone marrow cells that develop into white blood cells, including neutrophils, which help fight off infections.
FDA-approved products that may be used to treat adult and pediatric patients acutely exposed to myelosuppressive doses of radiation, a condition known as Hematopoietic Syndrome of Acute Radiation Syndrome, or H-ARS:
- Neupogen (filgrastim) – approved March 2015 [more info; product label (PDF, 1.2 MB)]
- Neulasta (pegfilgrastim) – approved November 2015 [product label (PDF, 1.7 MB)]
- Leukine (sargramostim) – approved March 29, 2018 [more info (PDF, 299 KB); product label (PDF, 786 KB)]
Venezuela: At least 68 people died when a fire broke out during a riot in the jail area of a police station
Thursday, March 29th, 2018Donors pledge over US$15 million to WHO’s Contingency Fund for Emergencies
Thursday, March 29th, 2018
27 March 2018 | Geneva – 27 March 2018 – Donors have pledged an additional US$15.3 million to support quick action by the World Health Organization to tackle disease outbreaks and humanitarian health crises through its emergency response fund in 2018, the Contingency Fund for Emergencies (CFE).
Canada, Denmark, Estonia, Germany, the Republic of Korea, Kuwait, Luxembourg, Malta, Netherlands, Norway, and the United Kingdom of Great Britain and Northern Ireland announced contributions ranging from US$20,000 to US$5.6 million at a conference hosted at WHO headquarters in Geneva, Switzerland on Monday (March 26) – increasing CFE funding levels to US$23 million.
This will enable the rapid financing of health response operations in the coming months – filling that critical gap between the moment the need for an emergency response is identified and the point at which funds from other sources can be released. WHO will seek to secure further donor commitments to achieve its US$100 million funding target for the 2018/2019 biennium.
First-time pledges were made by Denmark, Kuwait, Luxembourg, Malta and Norway. The UK has increased its overall commitment to the fund from US$10.5 million to US$16 million, making it the second largest donor after Germany.
“For the UK, the CFE is an extraordinarily good investment. We are convinced it has a vital and unique role to play in the global effort to prevent and mitigate health emergencies. Today we pledge an additional £4 million (US$5.6 million) for the Contingency Fund and pledge to work with WHO to better profile to a wider audience the huge value it brings. The G7 and the G20 share the UK’s desire for an adequately funded CFE. We urge our fellow Member States and donors to heed WHO’s call and to step forward to provide financial support for the Contingency Fund for Emergencies,” said Alistair Burt, UK Minister of State for International Development.
The CFE’s ability to release funds within 24 hours sets it apart from complementary financing mechanisms that have different funding criteria and slower disbursement cycles. While other funding mechanisms allow for the scale up of response operations, none are designed to deliver an immediate and early response. The CFE has demonstrated that a small investment can save lives and dramatically reduce the direct costs of controlling outbreaks and responding to emergencies.
“Without the CFE, recent outbreaks of Ebola in DRC, Marburg virus Disease in Uganda and pneumonic plague in Madagascar could have gotten out of control. By acting decisively and quickly, we can stop disease outbreaks and save thousands of lives for a fraction of the cost of a late response. The CFE has proven its value as a global public good that should be underwritten by long term investment,” said Dr Peter Salama, WHO Deputy Director General for Emergency Preparedness and Response.
Since 2015, the CFE has enabled WHO, national authorities and health partners to get quick starts on more than 50 disease outbreaks, humanitarian crises and natural disasters, allocating more than US$46 million. It has supported the rapid deployment of experts; better disease detection and reporting; the delivery of essential medicines, supplies and personal protective equipment; the strengthening of surveillance and vaccination; improved access to water, sanitation and health services; community engagement; and more.
Madagascar’s health minister Dr Lalatiana Andriamanarivo called for increased support for the CFE, saying it was instrumental to containing an unprecedented outbreak of pneumonic plague that rapidly spread across the island nation in 2017.
“We call on our international partners to support the Contingency Fund for Emergencies to enable WHO to respond to outbreaks everywhere across the world, and to reinforce national capacities to manage health emergencies in the future,” said Dr Andriamanarivo.
In 2017, the CFE provided nearly US$21 million for operations in 23 countries, with most allocations released within 24 hours. Over half (56%) of allocations funded responses in the WHO Africa region, with 28% going to responses in countries in the WHO Eastern Mediterranean Region and 11% to the South East Asia Region.
Note to editors
The WHO Health Emergencies Programme has three funding categories: the core budget that covers essential functions; the appeals budget that covers the additional work done in response to acute and protracted health emergencies; and the WHO Contingency Fund for Emergencies.
The Contingency Fund for Emergencies is replenished through donor contributions outside of the WHO Health Emergencies Programme core budget. Contributions are pooled and flexible, rather than earmarked for specific activities.
For more information, please contact:
Tarik Jašarević
Communications Officer
Telephone : +41 22 791 5099
Mobile: +41 793 676 214
E-mail: jasarevict@who.int


