Archive for the ‘Smallpox’ Category
Emergent BioSolutions today announced that it was awarded a contract worth about $2 billion over the next 10 years to deliver the ACAM200 smallpox vaccine to the Strategic National Stockpile.
Wednesday, September 4th, 2019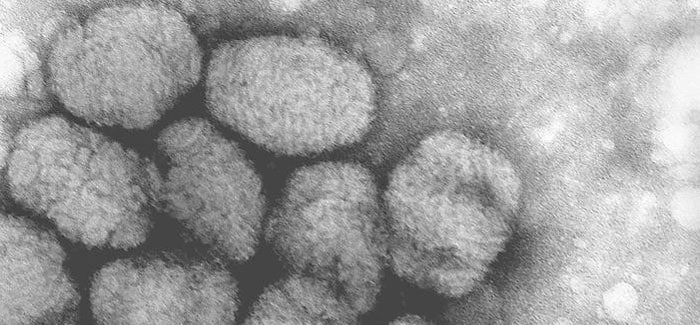
“…….About ACAM2000 Vaccine
ACAM2000 vaccine is the primary smallpox vaccine designated for use in a bioterrorism emergency, with almost 269 million doses having been supplied to the U.S. Strategic National Stockpile. ACAM2000 vaccine is also licensed in Australia and Singapore and is currently stockpiled both in the U.S. and internationally.
ACAM2000 is indicated for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection.
The labeling for ACAM2000 contains a contraindication for individuals with severe immunodeficiency. Severe localized or systemic infection with vaccinia (progressive vaccinia) may occur in persons with weakened immune systems. Individuals with severe immunodeficiency who are not expected to benefit from the vaccine should not receive ACAM2000. The risk for experiencing severe vaccination complications must be weighed against the risk for experiencing a potentially fatal smallpox infection.
Additionally, there are warnings and precautions for myocarditis, pericarditis, encephalitis, encephalomyelitis, encephalopathy, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson Syndrome), eczema vaccinatum resulting in permanent sequelae or death, ocular complications; blindness and fetal death have occurred following either primary vaccination or revaccination with live vaccinia virus smallpox vaccines. These risks are increased in certain individuals and may result in severe disability, permanent neurological sequalae and/or death.
Please see full Prescribing Information for full boxed warning and additional safety information…..”
Can Sydney, Australia manage a smallpox attack? It depends.
Friday, August 2nd, 2019MacIntyre CR, Costantino V, Kunasekaran MP (2019) Health system capacity in Sydney, Australia in the event of a biological attack with smallpox. PLoSONE 14(6): e0217704.https://doi. org/10.1371/journal.pone.0217704
“……We estimated 100638 clinical HCWs and 14595 public hospital beds in Sydney. Rapid response, case isolation and contact tracing are influential on epidemic size, with case isolation more influential than contact tracing. With 95% of cases isolated, outbreak control can be achieved within 100 days even with only 50% of contacts traced. However, if case isolation and contact tracing both fall to 50%, epidemic control is lost. With a smaller initial attack and a response commencing 20 days after the attack, health system impacts are modest. The requirement for hospital beds will vary from up to 4% to 100% of all available beds in best and worst case scenarios.
If the response is delayed, or if the attack infects 10000 people, all available beds will be exceeded within 40 days, with corresponding surge requirements for clinical health care workers (HCWs). We estimated there are 330 public health workers in Sydney with up to 940,350 contacts to be traced.
At least 3 million respirators will be needed for the first 100 days. To ensure adequate health system capacity, rapid response, high rates of case isolation, excellent contact tracing and vaccination, and protection of HCWs should be a priority. Surge capacity must be planned. Failures in any of these could cause health system failure, with inadequate beds, quarantine spaces, personnel, PPE and inability to manage other acute health conditions…..”
CDC: How to prepare for and respond to a smallpox emergency with smallpox vaccination how-to videos.
Monday, February 25th, 2019
FDA approves Tpoxx to fight smallpox
Saturday, July 14th, 2018“……never been tested in humans with smallpox because the disease was declared eradicated in 1980, three years after the last known case.
But it was very effective at protecting animals deliberately infected with monkeypox and rabbitpox, two related diseases that can be lethal. It also caused no severe side effects when safety-tested in 359 healthy human volunteers…..”
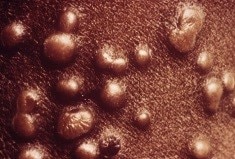
New study: Oral Tecovirimat for the Treatment of Smallpox
Sunday, July 8th, 2018“…..On the basis of its efficacy in two animal models and pharmacokinetic and safety data in humans, tecovirimat is being advanced as a therapy for smallpox in accordance with the FDA Animal Rule. …..”
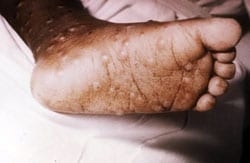
An FDA panel, comprised of independent medical experts, voted 17 to 0, that the benefits of TPOXX outweigh its risks as Smallpox antiviral therapy.
Thursday, May 3rd, 2018“….The U.S. government’s Biomedical Advanced Research and Development Authority (BARDA) funded the advanced development of oral TPOXX in partnership with SIGA. Additionally, under Project Bioshield, BARDA has acquired two million courses of oral TPOXX and such courses have been delivered to the Strategic National Stockpile (SNS)….”
SIGA Technologies, Inc. announces the submission of its New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for the oral formulation of TPOXX® (tecovirimat) to treat smallpox.
Wednesday, December 13th, 2017“…..If approved, the oral drug would be the first FDA-approved treatment for smallpox, a disease that has been eradicated but could be used as a bioterror weapon. The news comes on the heels of media reports that North Korea could be building a bioweapons program that might include smallpox.….”
NIH-appointed the seven-person blue ribbon panel to investigate the July 2014 discovery of 6 smallpox samples in an abandoned group of 300 lab samples that were in an unsecured Food and Drug Administration (FDA) cold-storage room during preparations for a move to the FDA’s main campus.
Tuesday, July 11th, 2017Final Document: Final_Report_of_the_Variola_Mishap-2017
In August of 2016, the National Institutes of Health (NIH) appointed a Blue Ribbon Panel to review the July 2014 discovery of six vials containing variola virus, the causative agent of smallpox, as well as over 300 other previously undiscovered biological samples on the NIH Bethesda, Maryland campus. The samples were found in an unsecured cold-storage room in a building occupied and managed by Food and Drug Administration (FDA) staff under NIH biosafety and biosecurity oversight.
The Panel includes a diverse group of seven external subject matter experts, and was constituted as a Working Group of the National Science Advisory Board for Biosecurity (NSABB), a federal advisory committee to the United States Government. The Panel began its work in August of 2016 and completed this final report in May of 2017. The Blue Ribbon Panel was charged with a number of tasks, including, 1) to determine how the smallpox virus vials came to be improperly stored and overlooked for years; 2) to identify any systemic issues that contributed to the lapse; and 3) to evaluate whether NIH had taken adequate corrective actions in response to this incident. It should be noted that this incident was one of several biosafety lapses involving federally regulated pathogens that occurred in the United States in 2014. These incidents caused considerable concern and led to substantial remedial activity throughout the United States Government.
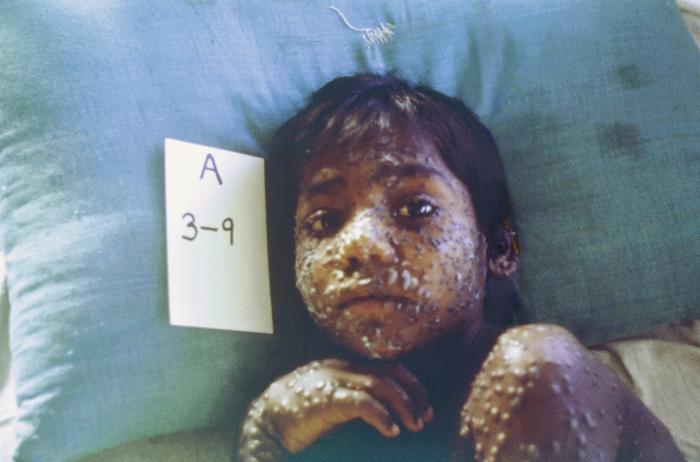
Smallpox was a devastating, contagious disease that infected over 300 million people in the 20th century, killing up to 30% of those infected. It was declared eradicated by the World Health Organization in 1980, and by international agreement, the remaining acknowledged samples were to be placed under tight control and oversight in only two repositories: in the US, at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia and in Russia, now at The State Research Center of Virology and Biotechnology (VECTOR), Novosibirsk.
While there are now smallpox vaccines available in the U.S. should they be needed, routine vaccination of Americans ceased in the mid-1970’s. As a result, nearly half of the population is unvaccinated, and, because vaccine effectiveness wanes over time, nearly the entire US population is potentially vulnerable to infection. The finding of viable smallpox virus samples outside of the established repositories was totally unanticipated and required an appropriate response and a rethinking of laboratory biosafety and biosecurity policy at NIH.
The Blue Ribbon Panel evaluation benefited from several prior comprehensive investigations of the smallpox virus incident, including by the Federal Bureau of Investigation jointly with the CDC, by the Government Accountability Office, and by Congress. The FDA also conducted an internal review. The Blue Ribbon Panel examined relevant federal and biosafety policy, regulation and guidance documents, as well as the many administrative actions taken by NIH in response to the 2014 smallpox virus discovery. The Panel visited pertinent sites on the NIH
3 | P a g e
campus, and interviewed those who had knowledge of or responsibility for the response. The Panel assessed that the incident has been now adequately evaluated and documented. Some questions remain, however, including the identity of the original owner of the samples and how they may have come to be in the cold-storage room. Unless further new information surfaces to solve this mystery, it is unlikely to be resolved, due to the passage of time. In addition, storage of the samples likely occurred before the Select Agents Program was established, at a time when biosafety standards were very different from today.
The Blue Ribbon Panel identified several key factors that contributed to the smallpox virus incident: There was a lack of individual responsibility for infectious materials in the FDA occupied space where the vials were found. There were also numerous missed opportunities to find the samples prior to 2014, particularly in the 1980’s when all smallpox virus samples were required to be either destroyed or sent to CDC, and again in 2003 when laws governing the possession of regulated pathogens, called “select agents,” were implemented. In addition, a lack of policies for dealing with abandoned research materials and for regular inventory of potentially hazardous biological materials were major contributors to the occurrence of this incident.
The panel assessed the response to the incident as appropriate and thorough, with excellent inter-agency cooperation (including the FBI) to manage a highly unusual situation. However, the Panel noted several specific problematic issues relating to the immediate response after discovery of the smallpox virus and other samples. In particular, the Panel determined that given the potentially hazardous nature of the abandoned samples, there are significant concerns about how they were packaged and transferred between buildings on the NIH campus. Following the discovery by an FDA official, it was decided by NIH that the samples should be immediately transferred to a secure high-containment Biosafety Level 3 laboratory. The samples, in their original boxes, were packaged into a larger cardboard box, and then handcarried to the secure NIH laboratory. No negative consequences occurred — there were no infections or injuries — but packaging and transport of the samples were conducted in ways that presented both biosafety and security risks. Other concerns identified by the panel included inadequate chain-of-custody and logging of events directly after the discovery of the vials, and the use of cardboard in cold-storage rooms. Although secondary to the issues associated with this event, cardboard containers can contribute to mold and unsanitary conditions.
The Panel assessed efforts pursued by NIH following the incident that were intended to improve biosafety and biosecurity procedures and minimize the likelihood of such occurrences in the future. It was determined that most of the factors and causative issues have been addressed by NIH’s subsequent efforts and policy revisions and are detailed in the report. However, there are several remaining issues requiring attention.
4 | P a g e
The Blue Ribbon Panel offers to NIH the following observations and recommendations. First, with regard to specific, detailed steps NIH should take to remedy remaining gaps in biosafety policies and procedures, the BRP recommends: Revise several specific biosafety policies and procedures, as detailed in the report. Rapidly finish the on-going space audit to ensure responsibility and oversight of all research materials is assigned to individuals by name, and updated as required by personnel changes. Ensure that any shared research space arrangements have clear written agreements with responsibilities well defined. The BRP noted that NIH policies and procedures use terms that are not in general use; such as “High consequence pathogens,” “and “potentially infectious materials. These categories need to be defined carefully or eliminated when not clearly necessary.
Second, regarding more general approaches to improving biosafety and biosecurity at NIH, the BRP considers the following to be important considerations:
Effective and complete implementation of current policies, procedures, guidance and practices on an on-going basis over time will be critical to ensuring safety and security surrounding pathogen research at NIH. The importance of leadership at the highest levels and continuous efforts to develop and maintain a culture of safety and responsibility among research staff cannot be overemphasized. There would be significant benefits to having consistent biosafety and biosecurity policies across the Department of Health Human Services (HHS) and the entire USG; ideally, insofar as possible, these should be harmonized with efforts by governments and international institutions as well. The BRP recommends continuing on-going efforts to address these issues. Response plans coordinated and exercised with agencies outside of HHS, including the Federal Bureau of Investigation, the Environmental Protection Agency and others, as needed, remain important. The variola virus incident illustrates how changes in infectious disease epidemiology and biosafety practices over time can radically alter a situation from “standard lab practice” to a potential major public health event.
The Blue Ribbon Panel concludes its effort with the observation that the smallpox virus incident illustrates how biosafety practices and laboratory management need to be continually evaluated. There was a time that research with smallpox virus could be conducted in relatively routine containment settings because the virus presented no extraordinary danger to the US population, as most everyone was vaccinated. As other infectious diseases are eradicated or controlled in the future, similar situations to the events of 2014 may arise — for example, with polio virus, which is currently close to eradication. Research samples and collections not
5 | P a g e
previously considered a significant biosecurity concern, need to be routinely re-evaluated to ensure proper biosafety and handling so that public health and safety are not compromised.
CDC’s Emergency Drugs for US Clinicians and Hospitals
Thursday, June 8th, 2017Our Formulary
The following information is provided as an informational resource for guidance only. It is not intended as a substitute for professional judgment. These highlights and any hyperlinks may not include all the information needed to use each respective drug or biologic safely and effectively. See full prescribing information (package insert) or IND protocol for each respective drug or biologic, which accompany the product when it is delivered to the treating physician and/or pharmacist.
The Drug Service formulary is subject to change based on current public health needs, updates to treatment guidelines, and/or drug availability. For historical reference, we have included products no longer supplied by the Drug Service.
| Product & Supplier | Indication & Eligibility | How Supplied |
|---|---|---|
| Anthrax Vaccine Absorbed
(Also known as “AVA”; BioThrax®, Emergent BioSolutions) |
For the active immunization for the prevention of disease caused by Bacillus anthracis, in persons 18 through 65 years of age at high risk for exposure
Because the risk for anthrax infection in the general population is low, routine immunization is not recommended The safety and efficacy of BioThrax® in a post-exposure setting have not been established. |
Suspension for injection in 5 mL multidose vials, each containing 10 doses |
| Artesunate, intravenous
(Supplied to CDC by the Walter Reed Army Institute of Research) |
For the treatment of severe malaria in patients who require parenteral (IV) therapy
Patient must meet the eligibility criteria in the IND protocol |
110 mg; sterile dry-filled powder with phosphate buffer diluent for reconstitution |
| Benznidazole
(Benznidazol, Manufactured by LAFEPE) |
For the treatment of American trypanosomiasis (Chagas disease)
Patient must meet the eligibility criteria in the IND protocol |
100 mg double-scored tablet
12.5 mg dispersible tablet for pediatric use |
| Botulism Antitoxin Heptavalent (Equine), Types A-G
(Also known as “HBAT”; Manufactured by Cangene Corp. – BAT™) |
For the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin | 20 mL or 50 mL single-use glass vial
May be received frozen or thawed |
| Diethylcarbamazine
(Also known as “DEC”; Supplied to CDC by the World Health Organization; Manufactured by E.I.P.I.C.O.) |
For the treatment of certain filarial diseases, including lymphatic filariasis caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori; tropical pulmonary eosinophilia; and loiasis
For prophylactic use in persons determined to be at increased risk for Loa loa infection Patient must meet the eligibility criteria in the IND protocol |
100 mg tablet |
| Diphtheria Antitoxin (Equine)
(Also known as “DAT”; Manufactured by Instituto Butantan) |
For prevention or treatment of actual or suspected cases of diphtheria
Patient must meet the eligibility criteria in the IND protocol |
1 mL single-use ampule containing 10,000 units |
| Eflornithine
(Also known as “DFMO”; Supplied to CDC by the World Health Organization; Manufactured by Sanofi Aventis – Ornidyl®) |
For the treatment of second-stage African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei gambiense, with involvement of the central nervous system | 20 g/100 mL hypertonic solution for IV infusion
Must be diluted with Sterile Water for Injection before use |
| Melarsoprol
(Supplied to CDC by the World Health Organization; Manufactured by Sanofi Aventis – Arsobal®) |
For the treatment of second-stage African trypanosomiasis (sleeping sickness), with involvement of the central nervous system
Patient must meet the eligibility criteria in the IND protocol |
5 mL glass ampule containing 180 mg/5 mL (36 mg/mL) |
| Nifurtimox
(Supplied to CDC by the World Health Organization; Manufactured by Bayer – Lampit®) |
For the treatment of American trypanosomiasis (Chagas disease)
Patient must meet the eligibility criteria in the IND protocol |
120 mg double-scored tablet |
| Sodium Stibogluconate
(Manufactured by GlaxoSmithKline, UK – Pentostam®) |
For the treatment of leishmaniasis
Patient must meet the eligibility criteria in the IND protocol |
Solution for injection in 100 mL multidose bottle
100 mg pentavalent antimony (Sb) per mL |
| Suramin
(Supplied to CDC by the World Health Organization; Manufactured by Bayer – Germanin) |
For the treatment of first-stage African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei rhodesiense, without involvement of the central nervous system
Patient must meet the eligibility criteria in the IND protocol |
1 gram of suramin for injection in a 10 mL vial (100 mg/mL solution of suramin sodium)
Must be reconstituted with 10 mL Sterile Water for Injection before use |
| Vaccinia Vaccine
(Also known as the “Smallpox Vaccine”; Manufactured by Sanofi Aventis – ACAM2000®) |
For active immunization against smallpox disease for persons determined to be at high risk for smallpox infection | Lyophilized powder reconstituted with diluent (provided)
Contains 100 doses per vial |
Anthrax Vaccine Adsorbed
Anthrax Vaccine Adsorbed (AVA) is indicated for the active immunization for the prevention of disease caused by Bacillus anthracis, in persons 18 through 65 years of age at high risk for exposure. The safety and efficacy of AVA in a post-exposure setting have not been established.
CDC provides anthrax vaccine for laboratory workers conducting research under federally funded projects who require preexposure vaccination based on their occupational risk.
Preexposure vaccination is recommended for laboratorians at risk for repeated exposure to fully virulent B. anthracis spores, such as those who 1) work with high concentrations of spores with potential for aerosol production; 2) handle environmental samples that might contain powders and are associated with anthrax investigations; 3) routinely work with pure cultures of B. anthracis; 4) frequently work in spore-contaminated areas after a bioterrorism attack; or 5) work in other settings where repeated exposures to B. anthracis aerosols may occur. Read more[PDF – 36 pages](https://www.cdc.gov/mmwr/pdf/rr/rr5906.pdf).
More Information for Clinicians
CDC’s Anthrax Vaccination Website
Educational Toolkit for Clinicians (from Department of Defense Anthrax Immunization Program)
Full Prescribing Information for BioThrax®
How to Request
Anthrax vaccine must be administered by or under the supervision of the physician who registers with CDC.
Contact the CDC Drug Service for more information.
Artesunate, Intravenous
Artesunate is in the class of medications known as artemesinins, which are derivatives from the “qinghaosu” or sweet wormwood plant (Artemisia annua). Artesunate is not currently licensed by FDA but is made available in the United States under a CDC-sponsored IND protocol for treatment of documented cases of severe malaria(https://www.cdc.gov/malaria/about/index.html) that require parenteral therapy. Read more(https://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html).
More Information for Clinicians
Diagnostic assistance for malaria is available through DPDx.
How to Request
Clinicians who wish to obtain artesunate for severe malaria should contact the CDC Malaria Hotline at 770-488-7788 (M-F, 8am-4:30pm, Eastern time) or, after hours, the CDC Emergency Operations Center (EOC) at 770-488-7100, and request to speak with a CDC Malaria Branch clinician. A Malaria Branch clinician will provide clinical consultation by telephone and, if indicated, authorize the emergency release of artesunate from one of the CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
Requests for unapproved uses cannot be granted.
For non-emergency questions related to artesunate IV, contact the CDC Drug Service.
Benznidazole
Benznidazole is a 2-nitroimidazole trypanocidal agent that was introduced in 1971 for the treatment of Trypanosoma cruzi infection—i.e., Chagas disease, also known as American trypanosomiasis(https://www.cdc.gov/parasites/chagas/). Benznidazole is one of two drugs available from CDC for the treatment of Chagas disease (the other is nifurtimox). In the United States, the need to have drugs available for treating Chagas disease has been increasing, largely because of implementation of T. cruzi blood-donor screening in 2007, which has identified chronically infected persons (mainly Latin American immigrants) who might benefit from treatment and has heightened awareness of Chagas disease.
More Information for Clinicians
Diagnostic assistance for American trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of Chagas disease should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email chagas@cdc.gov) M-F 7:30am-4pm EST.
For emergencies (for example, acute Chagas disease with severe manifestations, Chagas disease in a newborn, or Chagas disease in an immunocompromised person) outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Botulism Antitoxin Heptavalent (Equine), Types A-G
Botulism Antitoxin Heptavalent (HBAT) contains equine-derived antibody to the seven known botulinum toxin types (A-G). HBAT is composed of <2% intact immunoglobulin G (IgG) and ≥90% Fab and F(ab’)2 immunoglobulin fragments. These fragments are created by the enzymatic cleavage and removal of Fc immunoglobulin components in a process sometimes referred to as despeciation. HBAT is supplied on an emergency basis for the treatment of persons thought to be suffering from botulism and works by neutralizing unbound toxin molecules. In 2010, HBAT became the only botulism antitoxin available in the United States for naturally occurring non-infant botulism.
It is available only from CDC because of its limited use and its relatively short expiration date. The antitoxin is stored at CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
BabyBIG® (botulism immune globulin) remains available for infant botulism through the California Infant Botulism Treatment and Prevention Program.
More Information for Clinicians
How to Request
Clinicians who suspect a diagnosis of botulism in a patient should immediately call their state health department’s 24-hour telephone number(https://www.cdc.gov/mmwr/international/relres.html) to maintain effective botulism surveillance and to facilitate rapid detection of outbreaks. The state health department will contact CDC to arrange for a clinical consultation by telephone and, if indicated, release of botulism antitoxin. State health departments requesting botulism antitoxin should contact the CDC Emergency Operations Center (EOC) at 770-488-7100. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5232a8.htm).
For non-emergency questions concerning botulism antitoxin, contact the CDC Drug Service.
Diethylcarbamazine (DEC)
DEC is an antihelminthic agent used for treatment of lymphatic filariasis (caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori), tropical pulmonary eosinophilia, and loiasis(https://www.cdc.gov/parasites/loiasis/); DEC also has prophylactic benefit for Loa loa infection. DEC has been used worldwide for more than 50 years. In the past, Wyeth-Ayerst Laboratories made DEC available as a licensed drug; in the late 1990s, because of unavailability of a bulk chemical supplier, Wyeth-Ayerst discontinued distribution of DEC in the United States.
More Information for Clinicians
Diagnostic assistance for filarial diseases is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of filarial diseases should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
After-hours emergencies: 1-770-488-7100
Diphtheria Antitoxin (Equine)
Diphtheria antitoxin (DAT) is used to prevent or treat diphtheria by neutralizing the toxins produced by Corynebacterium diphtheriae. DAT is a sterile, aqueous solution of the refined and concentrated proteins, chiefly globulins, containing antibodies obtained from the serum of horses that have been immunized against diphtheria toxin. DAT is available under an IND protocol sponsored by CDC and is released only for actual or suspected cases of diphtheria(https://www.cdc.gov/diphtheria/about/index.html). The antitoxin is stored at CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
More Information for Clinicians
CDC’s Vaccine-Related Topics: Diphtheria Antitoxin(https://www.cdc.gov/diphtheria/dat.html)
How to Request
Clinicians who suspect a diagnosis of respiratory diphtheria can obtain DAT by contacting the Emergency Operations Center at 770-488-7100. They will be connected with the diphtheria duty officer, who will provide clinical consultation and, if indicated, initiate the release of diphtheria antitoxin.
For non-emergency questions concerning diphtheria antitoxin, contact the CDC Drug Service.
Eflornithine
Eflornithine is an antitrypanosomal agent that inhibits the enzyme ornithine decarboxylase. Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/); the choice of therapy depends on the infecting subspecies of the parasite and on the stage of the infection. Eflornithine is considered the drug of choice for the treatment of second-stage Trypanosoma brucei gambiense (West African) infection, with involvement of the central nervous system. It is not effective against T. b. rhodesiense (East African) infection (see melarsoprol). Although the manufacturer, Aventis, maintains its US licensure, eflornithine is not commercially available in the United States.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Melarsoprol
Melarsoprol is an organoarsenic compound with trypanocidal effects that has been used outside the United States since 1949. Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/); the choice of therapy depends on the infecting subspecies of the parasite and on the stage of the infection. Melarsoprol is used for the treatment of second-stage infection (involving the central nervous system). It is the only available therapy for second-stage Trypanosoma brucei rhodesiense (East African) infection, whereas eflornithine typically is used for second-stage T. b. gambiense (West African) infection.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Nifurtimox
Nifurtimox is a nitrofuran analog that was introduced in 1965 for the treatment of Trypanosoma cruzi infection—i.e., Chagas disease, also known as American trypanosomiasis(https://www.cdc.gov/parasites/chagas/). Nifurtimox is one of two drugs available from CDC for the treatment of Chagas disease (the other is benznidazole). In the United States, the need to have drugs available for treating Chagas disease has been increasing, largely because of implementation of T. cruzi blood-donor screening in 2007, which has identified chronically infected persons (mainly Latin American immigrants) who might benefit from treatment and has heightened awareness of Chagas disease.
More Information for Clinicians
Diagnostic assistance for American trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of Chagas disease should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email chagas@cdc.gov) M-F 7:30am-4pm EST.
For emergencies (for example, acute Chagas disease with severe manifestations, Chagas disease in a newborn, or Chagas disease in an immunocompromised person) outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Sodium Stibogluconate
Sodium stibogluconate (Pentostam®) is a pentavalent antimony compound used for treatment of leishmaniasis(https://www.cdc.gov/parasites/leishmaniasis). The three main clinical syndromes in humans are visceral, cutaneous, and mucosal leishmaniasis. Pentostam is a well-established antileishmanial agent that has been used in many countries of the world for more than half a century.
More Information for Clinicians
Diagnostic assistance for leishmaniasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of leishmaniasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
After-hours emergencies: 1-770-488-7100
Suramin
Suramin is a negatively charged, high-molecular-weight sulfated naphthylamine. It was introduced in the 1920s for the treatment of African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/). Suramin generally is considered the drug of choice for first-stage Trypanosoma brucei rhodesiense (East African) infection, without involvement of the central nervous system. Pentamidine typically is used for first-stage T. b. gambiense (West African) infection.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Vaccinia Vaccine, “Smallpox Vaccine”
Smallpox vaccine is made of live vaccinia virus derived from plaque purification cloning of Dryvax® (calf lymph vaccine, New York City Board of Health Strain) and grown in African Green Monkey kidney (Vero) cells and tested to be free of adventitious agents. It contains approximately 2.5 – 12.5 x 105 plaque-forming units per dose.
Smallpox was declared globally eradicated in 1980. In 1982, Wyeth Laboratories, the only active manufacturer of licensed vaccinia vaccine in the United States, discontinued production; and, in 1983, distribution to the civilian population was discontinued. Since January 1982, smallpox vaccination has not been required for international travelers, and International Certificates of Vaccination no longer include smallpox vaccination. ACAM2000® is a new-generation smallpox vaccine that was licensed in 2010 for use as a medical countermeasure held by the Strategic National Stockpile.
CDC recommends vaccinia vaccine for laboratory workers who directly handle a) cultures or b) animals contaminated or infected with nonhighly attenuated vaccinia virus, recombinant vaccinia viruses derived from nonhighly attenuated vaccinia strains, or other orthopoxviruses that infect humans (e.g., monkeypox, cowpox, vaccinia, and variola). Other health-care workers (e.g., physicians and nurses) whose contact with nonhighly attenuated vaccinia viruses is limited to contaminated materials (e.g., dressings) and who adhere to appropriate infection control measures are at lower risk for inadvertent infection than laboratory workers. However, because a theoretical risk for infection exists, vaccination can be offered to this group. Read more[PDF – 930KB](https://www.cdc.gov/mmwr/pdf/rr/rr5010.pdf).
More Information for Clinicians
Full Prescribing Information for ACAM2000®[PDF – 11 pages]
ACAM2000® Medication Guide[PDF – 6 pages]
CDC’s Vaccine-Related Topics: Smallpox Vaccine
How to Request
Smallpox vaccine must be administered by or under the supervision of the physician who registers with CDC.
Ancillary supplies, such as bifurcated needles (for administration) and 1 mL tuberculin syringes with 25 gauge x 5/8″ needles (for reconstitution), are supplied with the vaccine.
Contact the CDC Drug Service for more information.
Requests for unapproved uses cannot be granted.
Products No Longer Supplied by Drug Service*
Botulinum Toxoid
Pentavalent (ABCDE) botulinum toxoid is a combination of aluminum phosphate-adsorbed toxoid derived from formalin-inactivated types A, B, C, D, and E botulinum toxins, with formaldehyde and thimerosal used as preservatives. Botulinum toxoid was distributed by CDC under an IND protocol for at-risk persons who were actively working or expected to be working with cultures of Clostridium botulinum or the toxins; in 2011, CDC discontinued its program to supply this vaccine. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6042a3.htm).
Botulinum Antitoxin Types AB & E
In March 2010, CDC announced the availability of a new heptavalent botulinum antitoxin (HBAT, Cangene Corporation). HBAT replaced the licensed bivalent botulinum antitoxin AB and an investigational monovalent botulinum antitoxin E (BAT-AB and BAT-E, Sanofi Pasteur), becoming the only botulinum antitoxin available in the United States for naturally occurring non-infant botulism. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5910a4.htm).
Vaccinia Immune Globulin (VIG)
Vaccinia immune globulin (VIG) is released from the CDC Strategic National Stockpile, if indicated, for the treatment of complications associated with vaccinia vaccination. Clinicians wishing to obtain VIG should contact the Emergency Operations Center (EOC) at 770-488-7100. They will be connected with CDC medical staff who can assist them in the diagnosis and management of patients with suspected complications of vaccinia vaccination.
*this list is not all-inclusive
Use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
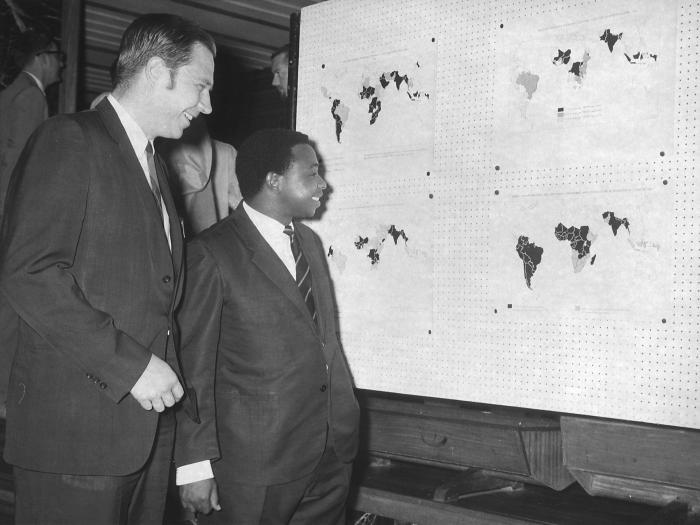
Dr. Donald A. Henderson, a leader of one of mankind’s greatest public health triumphs, the eradication of smallpox, died on Friday in Towson, Md.
Monday, August 22nd, 2016
This May, 1969 photograph was taken in the Ikoya Hotel in Lagos, Nigeria during a regional conference that same year focusing on the eradication of smallpox during worldwide eradication efforts.
Former Epidemic Intelligence Officer (EIS), and in 1966 head of the World Health Organization’s (WHO) Global Smallpox Eradication Campaign, D.A. Henderson, M.D. (foreground) was shown here as he discussed the geographically plotted effects that eradication efforts were having across the world. To Dr. Henderson’s left was the 1969, Nigerian Minister of Health.