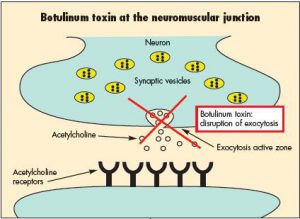
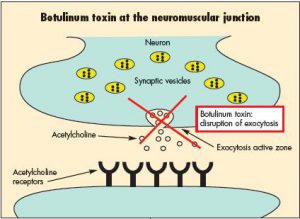
Archive for the ‘Botulism’ Category
CDC Video: Preparing and Administrating Heptavalent Antitoxin for the Treatment of Botulism
Friday, May 21st, 2021
Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR Recomm Rep 2021;70(No. RR-2):1–30. DOI: http://dx.doi.org/10.15585/mmwr.rr7002a1external icon
https://www.cdc.gov/mmwr/volumes/70/rr/rr7002a1.htm?ACSTrackingID=USCDC_1052-DM57269&ACSTrackingLabel=CDC%20Publishes%20First%20Comprehensive%20Clinical%20Guidelines%20for%20Diagnosis%20and%20Treatment%20of%20Botulism&deliveryName=USCDC_1052-DM57269
Infant Botulism: Information for Clinicians
Call ⇒
Infant Botulism Treatment and Prevention Program at the California Department of Public Health
Available 24/7
If you suspect your patient has botulism, call immediately. Do not wait for laboratory confirmation.
The on-call physician will provide a no-cost clinical consultation and release BabyBIGexternal icon treatment if the patient’s clinical findings indicate infant botulism.
Call your state health departmentexternal icon to report the suspected case.
Treat ⇒
Prepareexternal icon and administerexternal icon BabyBIG as soon as it is received. Do not wait for laboratory confirmation.
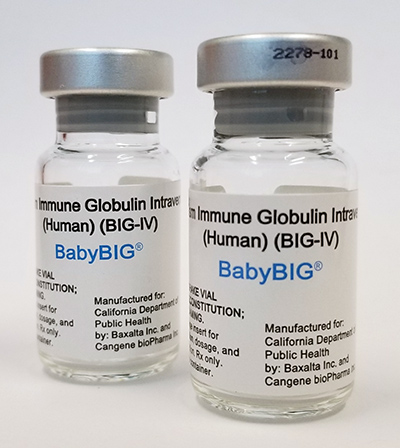
BabyBIG®, human antitoxin for the treatment of infant botulism.
Photo courtesy of the California Department of Public Health.
Test ⇒
Submit specimens for diagnostic testing through your state health department’s laboratory or CDC. All patients receiving BabyBIG must be tested.
Some laboratories, including CDC, do not routinely accept specimens unless BabyBIG has been released.
Questions about testing and reporting should be directed to your state health department.
Receive
The laboratory will report results and notify the state health department of positive results.
Testing can take several days. If you do not hear back within 5 days, contact your state health department.
Heptavalent Botulinum Antitoxin Treatment
Saturday, August 3rd, 2019Jason S Richardson, Geraldine S Parrera, Hugo Astacio, Harpreet Sahota, Deborah M Anderson, Christine Hall, Tim Babinchak, Safety and Clinical Outcomes of an Equine-Derived Heptavalent Botulinum Antitoxin Treatment for Confirmed or Suspected Botulism in the United States, Clinical Infectious Diseases, , ciz515, https://doi.org/10.1093/cid/ciz515
“…….Among 162 patients, 7 (4.3%) experienced BAT product-related serious adverse events, including 1 (0.6%) report each of pneumonia, pneumonia aspiration, ventricular tachycardia, upper gastrointestinal hemorrhage, anaphylactic reaction, acute kidney injury, and acute myocardial infarction. Thirty-one (19.1%) patients had 41 BAT product-related adverse events. Six (3.7%) deaths were reported in the registry. All deaths were attributed to the underlying illness and were assessed as unlikely related to BAT product. Among 113 (69.8%) patients with a final diagnosis of botulism, those treated early (≤2 days) spent fewer days in the hospital (5 vs 15.5 days), in the intensive care unit (4 vs 12 days), and on mechanical ventilation (6 vs 14.5 days) than those treated late (>2 days), respectively……..”
3 confimed cases of infant botulism in East Texas and there may be a 4th. Why? Chi lo sa!
Wednesday, July 17th, 2019‘……”We are certainly curious about where this originated from,” said Russell Hopkins, with Northeast Texas Public Health District.
According to Hopkins, two cases occurred in Troup, one in Mineola, and they are expecting a fourth case to be confirmed in Tyler.
“We’re not really sure what the commonality is, because they are kind of spread out,” Hopkins said. “You do have two cases in one town, which is unusual. But it doesn’t mean they’re related.”…..’

The Korea Centers for Disease Control and Prevention (KCDC) said it confirmed the first case of infant botulism
Thursday, June 20th, 2019“…..The center said its investigation was on a four months old infant in Jeonju, North Jeolla Province, who was diagnosed with infant botulism after showing symptoms of poor feeding and drooping eyelids in early June. The baby was hospitalized on June 4 and physicians requested a test by KCDC to confirm the diagnosis. The test results found Clostridium botulinum bacteria in the patient’s excrement on Monday.
The child is receiving stable treatment in a general ward and the KCDC provided botulinum antitoxin to the hospital to treat the patient.
KCDC said the center and North Jeolla Province dispatched epidemiologists to identify the infection routes and plan to secure additional samples from foods and residential environments for a precise analysis……”
NASA
Argentina: 2 cases of foodborne botulism linked to hummus have been confirmed.
Sunday, May 19th, 2019Scotland: Four wound botulism cases (3 confirmed and one probable) since February.
Thursday, April 25th, 2019“……All cases presented with clinical features consistent with wound botulism, and all involve people who inject drugs (PWID). There have been no deaths among the identified cases...…”

Quinta paciente contaminada pela bactéria Clostridium botulinum, causadora da doença de ‘Botulismo’.
Thursday, February 21st, 2019
Black tar heroin, skin popping: Wound botulism
Friday, January 4th, 2019Wound Botulism Outbreak Among Persons Who Use Black Tar Heroin — San Diego County, California, 2017–2018
Corey M. Peak, ScD; Hilary Rosen, MPH; Amanda Kamali, MD; et al.
“…….During September 2017–April 2018, nine cases of wound botulism were reported in San Diego County, California; all patients reported injecting heroin, and seven used black tar heroin, including subcutaneous injection in six patients. Symptoms were first attributed to drug intoxication for four patients; two received the opioid overdose reversal medication naloxone without improvement in symptoms. One patient died.
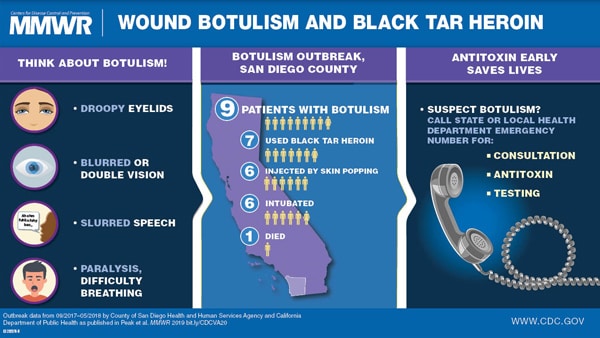
Increasing use of black tar heroin during the opioid crisis might lead to additional cases of wound botulism. Heightened awareness of the disease might improve timely diagnosis and treatment. Prompt diagnosis and administration of botulism antitoxin can be lifesaving….”
Texas: Mexican honey-filled pacifiers linked to 4 infant botulism cases
Monday, November 19th, 2018“…… four children from around the state came down with infant botulism from August to October, and they all were reported to have been given honey pacifiers purchased in Mexico…….”
Infant botulism cases prompt alert about honey pacifiers
News Release
Nov. 16, 2018
Children under 12 months old should not consume honey
The Texas Department of State Health Services is warning parents and other adults not to give babies pacifiers containing honey after four babies were treated for botulism in Texas. Each infant had been given a honey-containing pacifier purchased in Mexico.
The four illnesses occurred from mid-August to the end of October and caused all four babies to be hospitalized for life-saving treatment. The unrelated infants are residents of West Texas, North Texas and South Texas.
Botulism is a serious illness caused by a toxin that attacks the body’s nerves and can cause difficulty breathing, paralysis and even death. Honey may contain bacteria that produce the toxin in the intestine of babies that eat it. By the time children get to be 12 months old, they’ve developed enough other types of bacteria in their digestive tract to prevent the botulism bacteria from growing and producing toxin.
DSHS today also issued a health alert asking health care providers to look out for cases of infant botulism and to remind parents not to let babies eat honey. The Centers for Disease Control and Prevention and the American Academy of Pediatrics have long advised that children under 12 months old should not consume honey.Honey-filled pacifiers are not common in the United States but may be available in some specialty stores and through online retailers. Most aren’t designed for the honey to be consumed, but some have a small hole so a child could eat the honey, or the pacifier could accidentally rupture or leak. Parents should also avoid pacifiers containing any other food substance, because they could also pose a risk of botulism.
Texas has had seven to eight cases of infant botulism per year in recent years. Ten confirmed or suspected cases have been reported in 2018. Additional information on botulism is available on the DSHS website.
-30-
Since the beginning of 2018 in Ukraine botulism has sickened 80 people, of whom 7 have died.
Sunday, August 19th, 2018
“…..Experts urged Ukrainians not to consume dried, salted and canned fish, canned food or meat products….”