CDC: Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine — United States, December 21, 2020–January 10, 2021.
January 22nd, 2021Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine — United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. ePub: 22 January 2021. DOI: http://dx.doi.org/10.15585/mmwr.mm7004e1external icon
Abbreviations: COVID-19 = coronavirus disease 2019; ED = emergency department; Epi = epinephrine; F = female.
* As documented in the VAERS report or medical records, or through confirmation with the treating health care provider or the patients themselves.
† Inpatient hospitalization.
§ The Brighton Collaboration case definition uses combinations of symptoms to define levels of diagnostic certainty. Brighton level 1 represents the highest level of
diagnostic certainty that a reported case is indeed a case of anaphylaxis; levels 2 and 3 are successively lower levels of diagnostic certainty. Level 4 is a case reported
as anaphylaxis but that does not meet the Brighton Collaboration case definition. Level 5 is a case that was neither reported as anaphylaxis nor meets the case
definition (https://doi.org/10.1016/j.vaccine.2007.02.064).
¶ As documented in the description of the adverse event in the VAERS report in Box 18 or as documented in recovery status in Box 20.
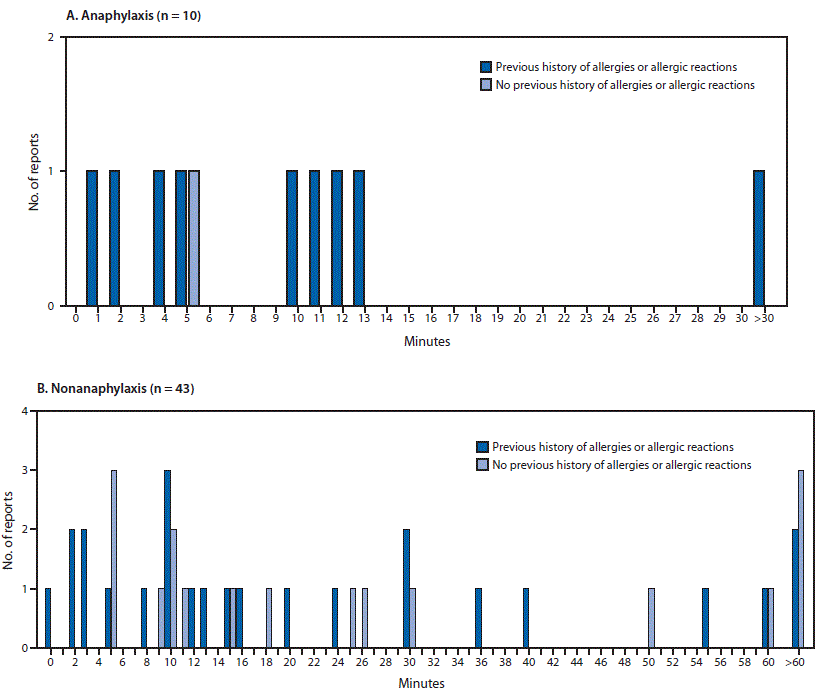
Minutes from vaccine receipt to onset of anaphylaxis (A)* and nonanaphylaxis allergic reactions (B)† after receipt of the first dose of Moderna COVID-19 vaccine — Vaccine Adverse Events Reporting System (VAERS), United States, December 21, 2020–January 10, 2021
Abbreviation: COVID-19 = coronavirus disease 2019.
* The interval from vaccine receipt to symptom onset was >30 minutes for one anaphylaxis case (45 minutes).
† The interval from vaccine receipt to symptom onset was ≥60 minutes for three nonanaphylaxis patients who had a documented history of allergies or allergic reactions at 60, 90, and 98 minutes and for four who did not have a documented history of allergies or allergic reactions (60 minutes, 10 hours, 20 hours, and 24 hours). The interval from vaccine receipt to symptom onset was missing in two case reports, both of which documented a history of allergies or allergic reactions. Four cases of nonanaphylaxis allergic reactions with symptom onset occurring later than the day after vaccination (i.e., outside of the 0–1-day risk window) were excluded from the final analysis.
Abbreviation: COVID-19 = coronavirus disease 2019.
* Four of the initial 47 nonanaphylaxis allergic reaction reports were excluded from the final analysis because symptom onset occurred later than the day after vaccination (i.e., outside the 0–1-day risk window).
† Two nonanaphylaxis allergic reaction reports were missing information on time of symptom onset; percentage calculated among 41 case reports with onset documented.
§ Five anaphylaxis reports included a patient history of a previous anaphylaxis episode.

