CDC: Coronavirus Disease 2019 (COVID-19) Situation Summary, 2/25/20
February 25th, 2020https://www.cdc.gov/coronavirus/2019-nCoV/summary.html
This is an emerging, rapidly evolving situation and CDC will provide updated information as it becomes available, in addition to updated guidance.
Updated February 25, 2020
Background
CDC is responding to an outbreak of respiratory disease caused by a novel (new) coronavirus that was first detected in Wuhan City, Hubei Province, China and which has now been detected in 37 locations internationally, including cases in the United States. The virus has been named “SARS-CoV-2” and the disease it causes has been named “coronavirus disease 2019” (abbreviated “COVID-19”).
On January 30, 2020, the International Health Regulations Emergency Committee of the World Health Organization declared the outbreak a “public health emergency of international concernexternal icon” (PHEIC). On January 31, 2020, Health and Human Services Secretary Alex M. Azar II declared a public health emergency (PHE) for the United States to aid the nation’s healthcare community in responding to COVID-19.
Source and Spread of the Virus
Coronaviruses are a large family of viruses that are common in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with MERS-CoV, SARS-CoV, and now with this new virus (named SARS-CoV-2).
The SARS-CoV-2 virus is a betacoronavirus, like MERS-CoV and SARS-CoV. All three of these viruses have their origins in bats. The sequences from U.S. patients are similar to the one that China initially posted, suggesting a likely single, recent emergence of this virus from an animal reservoir.
Early on, many of the patients in the COVID-19 outbreak in Wuhan, China had some link to a large seafood and live animal market, suggesting animal-to-person spread. Later, a growing number of patients reportedly did not have exposure to animal markets, indicating person-to-person spread. Person-to-person spread has been reported outside China, including in the United States and other locations. Chinese officials report that sustained person-to-person spread in the community is occurring in China. In addition, other destinations have apparent community spread, meaning some people have been infected who are not sure how or where they became infected. Learn what is known about the spread of newly emerged coronaviruses.
Situation in U.S.
Imported cases of COVID-19 in travelers have been detected in the U.S. Person-to-person spread of COVID-19 also has been seen among close contacts of returned travelers from Wuhan, but at this time, this virus is NOT currently spreading in the community in the United States.
Illness Severity
Both MERS-CoV and SARS-CoV have been known to cause severe illness in people. The complete clinical picture with regard to COVID-19 is not fully understood. Reported illnesses have ranged from mild to severe, including illness resulting in death. Learn more about the symptoms associated with COVID-19.
There are ongoing investigations to learn more. This is a rapidly evolving situation and information will be updated as it becomes available.
Risk Assessment
Outbreaks of novel virus infections among people are always of public health concern. The risk from these outbreaks depends on characteristics of the virus, including how well it spreads between people, the severity of resulting illness, and the medical or other measures available to control the impact of the virus (for example, vaccine or treatment medications). The fact that this disease has caused illness, including illness resulting in death, and sustained person-to-person spread is concerning. These factors meet two of the criteria of a pandemic. As community spread is detected in more and more countries, the world moves closer toward meeting the third criteria, worldwide spread of the new virus.
The potential public health threat posed by COVID-19 is high, both globally and to the United States.
But individual risk is dependent on exposure.
- For the general American public, who are unlikely to be exposed to this virus at this time, the immediate health risk from COVID-19 is considered low.
- Under current circumstances, certain people will have an increased risk of infection, for example healthcare workers caring for patients with COVID-19 and other close contacts of persons with COVID-19. CDC has developed guidance to help in the risk assessment and management of people with potential exposures to COVID-19.
However, it’s important to note that current global circumstances suggest it is likely that this virus will cause a pandemic. In that case, the risk assessment would be different.
What May Happen
More cases are likely to be identified in the coming days, including more cases in the United States. It’s also likely that person-to-person spread will continue to occur, including in the United States. Widespread transmission of COVID-19 in the United States would translate into large numbers of people needing medical care at the same time. Schools, childcare centers, workplaces, and other places for mass gatherings may experience more absenteeism. Public health and healthcare systems may become overloaded, with elevated rates of hospitalizations and deaths. Other critical infrastructure, such as law enforcement, emergency medical services, and transportation industry may also be affected. Health care providers and hospitals may be overwhelmed. At this time, there is no vaccine to protect against COVID-19 and no medications approved to treat it. Nonpharmaceutical interventions would be the most important response strategy.
CDC Response
Global efforts at this time are focused concurrently on containing spread of this virus and mitigating the impact of this virus. The federal government is working closely with state, local, tribal, and territorial partners, as well as public health partners, to respond to this public health threat. The public health response is multi-layered, with the goal of detecting and minimizing introductions of this virus in the United States so as to reduce the spread and the impact of this virus. CDC is operationalizing all of its pandemic preparedness and response plans, working on multiple fronts to meet these goals, including specific measures to prepare communities to respond local transmission of the virus that causes COVID-19. There is an abundance of pandemic guidance developed in anticipation of an influenza pandemic that is being repurposed and adapted for a COVID-19 pandemic.
Highlights of CDC’s Response
- CDC established a COVID-19 Incident Management System on January 7, 2020. On January 21, CDC activated its Emergency Operations Center to better provide ongoing support to the COVID-19 response.
- The U.S. government has taken unprecedented steps with respect to travel in response to the growing public health threat posed by this new coronavirus:
- Effective February 2, at 5pm, the U.S. government suspended entry of foreign nationals who have been in China within the past 14 days.
- U.S. citizens, residents, and their immediate family members who have been in Hubei province and other parts of mainland China are allowed to enter the United States, but they are subject to health monitoring and possible quarantine for up to 14 days.
- CDC has issued the following travel guidance related to COVID-19:
- China — Level 3, Avoid Nonessential Travel — updated February 22;
- South Korea — Level 3, Avoid Nonessential Travel — updated February 24;
- Japan — Level 2, Practice Enhanced Precautions — updated February 22;
- Iran — Level 2, Practice Enhanced Precautions — issued February 23;
- Italy — Level 2, Practice Enhanced Precautions — issued February 23;
- Hong Kong — Level 1, Practice Usual Precautions — issued February 19.
- CDC also recommends that all travelers reconsider cruise ship voyages into or within Asia at this time.
- CDC is issuing clinical guidance, including:
- An interim Health Alert Network (HAN) Update to inform state and local health departments and healthcare professionals about this outbreak on February 1.
- On January 30, CDC published guidance for healthcare professionals on the clinical care of COVID-19 patients.
- On February 3, CDC posted guidance for assessing the potential risk for various exposures to COVID-19 and managing those people appropriately.
- CDC has deployed multidisciplinary teams to support state health departments with clinical management, contact tracing, and communications.
CDC has worked with the Department of State, supporting the safe return of Americans who have been stranded as a result of the ongoing outbreaks of COVID-19 and related travel restrictions. CDC has worked to assess the health of passengers as they return to the United States and provided continued daily monitoring of people who are quarantined.

This is a picture of CDC’s laboratory test kit for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). CDC is shipping the test kits to laboratories CDC has designated as qualified, including U.S. state and local public health laboratories, Department of Defense (DOD) laboratories and select international laboratories. The test kits are bolstering global laboratory capacity for detecting SARS-CoV-2.
- CDC laboratories have supported the COVID-19 response, including:
- CDC has developed a real time Reverse Transcription-Polymerase Chain Reaction (rRT-PCR) test that can diagnose COVID-19 in respiratory samples from clinical specimens. On January 24, CDC publicly posted the assay protocol for this test.
- CDC has been uploading the entire genome of the viruses from reported cases in the United States to GenBank as sequencing was completed.
- CDC has grown the COVID-19 virus in cell culture, which is necessary for further studies, including for additional genetic characterization. The cell-grown virus was sent to NIH’s BEI Resources Repositoryexternal icon for use by the broad scientific community.
CDC Recommends
- While the immediate risk of this new virus to the American public is believed to be low at this time, everyone can do their part to help us respond to this emerging public health threat:
- It’s currently flu and respiratory disease season and CDC recommends getting a flu vaccine, taking everyday preventive actions to help stop the spread of germs, and taking flu antivirals if prescribed.
- If you are a healthcare provider, be on the look-out for people who recently traveled from China and have fever and respiratory symptoms.
- If you are a healthcare provider caring for a COVID-19 patient or a public health responder, please take care of yourself and follow recommended infection control procedures.
- If you have been in China or have been exposed to someone sick with COVID-19 in the last 14 days, you will face some limitations on your movement and activity. Please follow instructions during this time. Your cooperation is integral to the ongoing public health response to try to slow spread of this virus. If you develop COVID-19 symptoms, contact your healthcare provider, and tell them about your symptoms and your travel or exposure to a COVID-19 patient.
- For people who are ill with COVID-19, please follow CDC guidance on how to reduce the risk of spreading your illness to others.
Other Available Resources
The following resources are available with information on COVID-19
Locations with Confirmed COVID-19 Cases
February 25th, 2020Global Map
As of 11:00 a.m. ET February 25, 2020
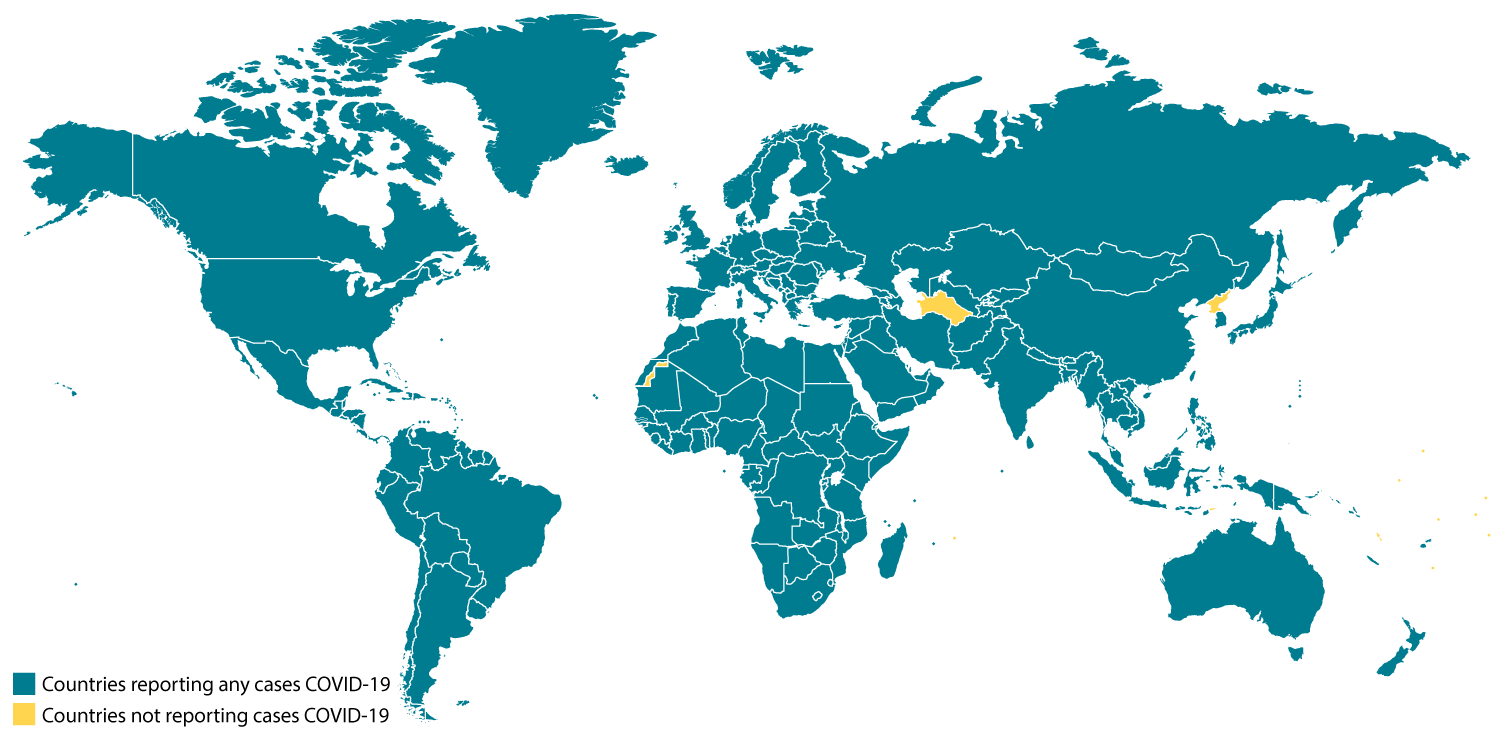
Locations with Confirmed COVID-19 Cases
- China
- Hong Kong
- Macau
- Taiwan
- Afghanistan
- Australia
- Bahrain
- Belgium
- Cambodia
- Canada
- Egypt
- Finland
- France
- Germany
- India
- Iran
- Iraq
- Israel
- Italy
- Japan
- Kuwait
- Lebanon
- Malaysia
- Nepal
- Oman
- Philippines
- Russia
- Sri Lanka
- Singapore
- Spain
- Sweden
- Thailand
- The Republic of Korea
- United Arab Emirates
- United Kingdom
- United States
- Vietnam
CROATIA, AUSTRIA, AND SWITZERLAND REPORT FIRST CASES of COVID-19
February 25th, 2020Austria has confirmed its first two cases of COVID-19 in Tyrol. Both cases have previous travel to Italy’s Lombardy region which is seeing increases in cases.
Switzerland also confirmed its first coronavirus case. The case was identified in canton Ticino in the southern part of the country, which borders Italy. The infected individual was reported to have been infected around February 15 in the Milan region of Italy.
Reuters has reported that Croatia has confirmed its first case of COVID-19. According to the news report, Prime Minister Andrej Plenkovic specified that the person is younger and with mild symptoms. In a previous press conference published, Health Minister Vili Beros discussed increased screening measures and questioning for people entering Croatia at border checkpoints. Foreigners reporting symptoms will be turned away. Additionally, quarantine measures would be implemented for people with a history of possible exposure.

CDC recommends that travelers avoid all nonessential travel to the People’s Republic of China
February 25th, 2020https://wwwnc.cdc.gov/travel/destinations/traveler/none/china
ChinaTraveler View
Travel warning: CDC recommends that travelers avoid all nonessential travel to the People’s Republic of China (this does not include Hong Kong, Macau, or the island of Taiwan).
Warning Level 3, Avoid Nonessential Travel
- Updated Novel Coronavirus in China February 22, 2020 CDC recommends that travelers avoid all nonessential travel to the People’s Republic of China (this does not include Hong Kong, Macau, or the island of Taiwan).
Coronavirus Disease 2019 Information for Travel
February 25th, 2020https://www.cdc.gov/coronavirus/2019-ncov/travelers/index.html
This page includes information about Coronavirus Disease 2019 (COVID-19) for travelers and travel related industries.
CDC recommends that travelers avoid all nonessential travel to:
These destinations are experiencing sustained community transmission of respiratory illness caused by the novel coronavirus (COVID-19). The virus can spread from person to person. Older adults and those with chronic medical conditions should consider postponing nonessential travel.
CDC does not recommend canceling or postponing travel to the following destinations. Travelers should practice usual precautions.
- Singapore
- Taiwan
- Thailand
- Vietnam
Community spread means people have been infected with the virus, including some who are not sure how or where they became infected. At this time, the extent of virus spread is not sustained or widespread enough to meet the criteria for a travel health notice. If that changes, CDC will update this page.
Resources for Ships
Resources for Airlines
CDC Travelers’ Health Update: 2/25/20
February 25th, 2020Coronavirus Disease 2019
Travel: Because of the novel (new) coronavirus outbreak, CDC recommends that travelers avoid travel to mainland China, South Korea, and reconsider cruise ship travel to or within Asia. The recommendation to avoid travel to China applies only to mainland China and does not include Hong Kong, Macau, or the island of Taiwan. CDC has posted several country-specific travel health notices for coronavirus. These notices are also listed in the travel health notice section below. The coronavirus situation is evolving rapidly, and CDC is following it very closely.
A randomized, controlled clinical trial to evaluate the safety and efficacy of the investigational antiviral remdesivir in hospitalized adults diagnosed with coronavirus disease 2019 (COVID-19) has begun at the University of Nebraska Medical Center (UNMC) in Omaha.
February 25th, 2020NIH
“……Remdesivir, developed by Gilead Sciences Inc., is an investigational broad-spectrum antiviral treatment. It was previously tested in humans with Ebola virus disease and has shown promise in animal models for treating Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), which are caused by other coronaviruses.
“We urgently need a safe and effective treatment for COVID-19. Although remdesivir has been administered to some patients with COVID-19, we do not have solid data to indicate it can improve clinical outcomes,” said NIAID Director and U.S. Coronavirus Task Force member Anthony S. Fauci, M.D. “A randomized, placebo-controlled trial is the gold standard for determining if an experimental treatment can benefit patients.”
Clinical trials of remdesivir are also ongoing in China. NIAID developed the current study taking those designs into account, and in accordance with consultations convened by the WHO on the development of a therapeutic trial for patients with COVID-19.
Participants in the NIH-sponsored trial must have laboratory-confirmed SARS-CoV-2 infection and evidence of lung involvement, including rattling sounds when breathing (rales) with a need for supplemental oxygen or abnormal chest X-rays, or illness requiring mechanical ventilation. Individuals with confirmed infection who have mild, cold-like symptoms or no apparent symptoms will not be included in the study. In accordance with standard clinical research protocols, eligible patients will provide informed consent to participate in the trial.
All potential participants will undergo a baseline physical exam before receiving treatment. Eligible study participants will then be randomly assigned either to the investigational treatment group or the placebo group. The study is double-blind, meaning trial investigators and participants would not know who is receiving remdesivir or placebo. Participants in the investigational treatment group will receive 200 milligrams (mg) of remdesivir intravenously on the first day of enrollment to the study. They will receive another 100 mg each day for the duration of hospitalization, for up to 10 days total. The placebo group will receive, at an equal volume, a solution that resembles remdesivir but contains only inactive ingredients.
Clinicians will regularly monitor participants and will assign them daily scores based on a predefined scale of clinical outcomes that considers factors such as temperature, blood pressure and use of supplemental oxygen, among others. Participants also will be asked to provide blood samples and nose and throat swabs approximately every two days. Researchers will test these specimens for SARS-CoV-2.
Initially, investigators will compare participant outcomes on day 15 in both the remdesivir group and the placebo group to see if the investigational drug increased clinical benefit compared to placebo. Outcomes are scored on a seven-point scale ranging from fully recovered to death. Investigators will reevaluate this scale after reviewing data from the first 100 participants.
An independent data and safety monitoring board (DSMB) will monitor ongoing results to ensure patient well-being and safety as well as study integrity. The DSMB will recommend the study be halted if there is clear and substantial evidence of a treatment difference between drug and placebo.
Andre Kalil, M.D., professor of internal medicine at UNMC and an infectious diseases physician at Nebraska Medicine, is leading the trial at UNMC. Thirteen people repatriated by the U.S. State Department from the Diamond Princess cruise ship were transported to the National Quarantine Unit, located within the Training, Simulation and Quarantine Center on the UNMC/Nebraska Medicine campus in Omaha on February 17, 2020. The passengers were in a close setting where there had been significant spread of COVID-19 and were sent to the unit for continued isolation and possibly further care. The CDC has since reported that 11 people in the UNMC unit have confirmed SARS-CoV-2 infection.
UNMC’s National Quarantine Unit is supported by the office of the Assistant Secretary for Preparedness and Response (ASPR) at the Department of Health and Human Services. It has a 20-bed capacity and is in close proximity to the Nebraska Biocontainment Unit, should a higher level of care be needed. Clinical trial participants are cared for in the biocontainment unit….”
A hotel in Tenerife in Spain’s Canary Islands has been locked down after a visiting Italian doctor tested positive for coronavirus.
February 25th, 2020BBC: https://www.bbc.com/news/world-europe-51627597
“…..Hundreds of guests at the H10 Costa Adeje Palace Hotel were initially told to stay in their rooms as medical tests were carried out, Spanish media report.
The doctor is reportedly from the Lombardy region, where Italian authorities are battling an outbreak……”
NASA
Italy is struggling to understand how it went from six coronavirus cases to more than 200 since last Friday
February 25th, 2020BBC: https://www.bbc.com/news/world-europe-51628084
“…So far, seven people have died.
“Patient zero” – the individual first infected – has still not been identified….”