CDC Confirms 14th Case of 2019 Novel Coronavirus
February 13th, 2020For Immediate Release: Wednesday, February 12, 2020
Contact: Media Relations
(404) 639-3286
CDC today confirmed another infection with 2019 novel coronavirus (COVID-19) in the United States in California. The patient is among a group of people under a federal quarantine order because of their recent return to the U.S. on a State Department-chartered flight that arrived on February 7, 2020.
All people who have been in Hubei Province in the past 14 days are considered at high risk of having been exposed to COVID-19 and subject to a temporary 14-day quarantine. This is the second person at this base who has tested positive for COVID-19. The first and second patients arrived on different planes and were housed in separate facilities; there are no epidemiologic links between them.
According to CDC on-site team lead Dr. Chris Braden, “At this time there is no indication of person-to-person spread of this virus at the quarantine facility, but CDC will carry out a thorough contact investigation as part of its current response strategy to detect and contain any cases of infection with this virus.”
This brings the total of number of COVID-19 cases in the United States to 14. There are likely to be additional cases in the coming days and weeks, including among other people recently returned from Wuhan. While 195 people were discharged from quarantine yesterday, more than 600 people who returned on chartered flights from Wuhan remain under federal quarantine.
For the latest information on the outbreak, visit CDC’s Novel Coronavirus 2019 website.
###
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICESexternal icon
CDC works 24/7 protecting America’s health, safety and security. Whether disease start at home or abroad, are curable or preventable, chronic or acute, or from human activity or deliberate attack, CDC responds to America’s most pressing health threats. CDC is headquartered in Atlanta and has experts located throughout the United States and the world.
CDC: Frequently Asked Questions about Respirators and their Use
February 11th, 2020This document is intended to address frequently asked questions about respirators and their use.
Respiratory Protection
- Should I wear a respirator in public?
- CDC does not recommend the routine use of respirators outside of workplace settings (in the community). Most often, spread of respiratory viruses from person-to-person happens among close contacts (within 6 feet). CDC recommends everyday preventive actions to prevent the spread of respiratory viruses, such as avoiding people who are sick, avoiding touching your eyes or nose, and covering your cough or sneeze with a tissue. People who are sick should stay home and not go into crowded public places or visit people in hospitals. Workers who are sick should follow CDC guidelines and stay home when they are sick.
- What is a respirator?
- A respirator is a personal protective device that is worn on the face or head and covers at least the nose and mouth. A respirator is used to reduce the wearer’s risk of inhaling hazardous airborne particles (including infectious agents), gases or vapors. Respirators, including those intended for use in healthcare settings, are certified by the CDC/NIOSH.
- What is an N95 filtering facepiece respirator (FFR)?
- An N95 FFR is a type of respirator which removes particles from the air that are breathed through it. These respirators filter out at least 95% of very small (0.3 micron) particles. N95 FFRs are capable of filtering out all types of particles, including bacteria and viruses.
- What makes N95 respirators different from facemasks (sometimes called a surgical mask)?
- Infographic: Understanding the difference between surgical masks and N95 respiratorspdf icon
- N95 respirators reduce the wearer’s exposure to airborne particles, from small particle aerosols to large droplets. N95 respirators are tight-fitting respirators that filter out at least 95% of particles in the air, including large and small particles.
- Not everyone is able to wear a respirator due to medical conditions that may be made worse when breathing through a respirator. Before using a respirator or getting fit-tested, workers must have a medical evaluation to make sure that they are able to wear a respirator safely.
- Achieving an adequate seal to the face is essential. United States regulations require that workers undergo an annual fit test and conduct a user seal check each time the respirator is used. Workers must pass a fit test to confirm a proper seal before using a respirator in the workplace.
- When properly fitted and worn, minimal leakage occurs around edges of the respirator when the user inhales. This means almost all of the air is directed through the filter media.
- Unlike NIOSH-approved N95s, facemasks are loose-fitting and provide only barrier protection against droplets, including large respiratory particles. No fit testing or seal check is necessary with facemasks. Most facemasks do not effectively filter small particles from the air and do not prevent leakage around the edge of the mask when the user inhales.
- The role of facemasks is for patient source control, to prevent contamination of the surrounding area when a person coughs or sneezes. Patients with confirmed or suspected 2019-nCoV should wear a facemask until they are isolated in a hospital or at home. The patient does not need to wear a facemask while isolated.
- What is a Surgical N95 respirator and who needs to wear it?
- A surgical N95 (also referred as a medical respirator) is recommended only for use by healthcare personnel (HCP) who need protection from both airborne and fluid hazards (e.g., splashes, sprays). These respirators are not used or needed outside of healthcare settings. In times of shortage, only HCP who are working in a sterile field or who may be exposed to high velocity splashes, sprays, or splatters of blood or body fluids should wear these respirators, such as in operative or procedural settings. Most HCP caring for confirmed or suspected 2019-nCoV patients should not need to use surgical N95 respirators and can use standard N95 respirators.
- If a surgical N95 is not available for use in operative or procedural settings, then an unvalved N95 respirator may be used with a faceshield to help block high velocity streams of blood and body fluids.
- My employees complain that Surgical N95 respirators are hot and uncomfortable – what can I do?
- The requirements for surgical N95 respirators that make them resistant to high velocity streams of body fluids and help protect the sterile field can result in a design that has a higher breathing resistance (makes it more difficult to breath) than a typical N95 respirator. Also, surgical N95 respirators are designed without exhalation valves which are sometimes perceived as warmer inside the mask than typical N95 respirators. If you are receiving complaints, you may consider having employees who are not doing surgery, not working in a sterile field, or not potentially exposed to high velocity streams of body fluids wear a standard N95 with an exhalation valve.
- My N95 respirator has an exhalation valve, is that okay?
- An N95 respirator with an exhalation valve does provide the same level of protection to the wearer as one that does not have a valve. The presence of an exhalation valve reduces exhalation resistance, which makes it easier to breathe (exhale). Some users feel that a respirator with an exhalation valve keeps the face cooler and reduces moisture build up inside the facepiece. However, respirators with exhalation valves should not be used in situations where a sterile field must be maintained (e.g., during an invasive procedure in an operating or procedure room) because the exhalation valve allows unfiltered exhaled air to escape into the sterile field.
CDC: Flowchart to Identify and Assess 2019 Novel Coronavirus
February 11th, 2020
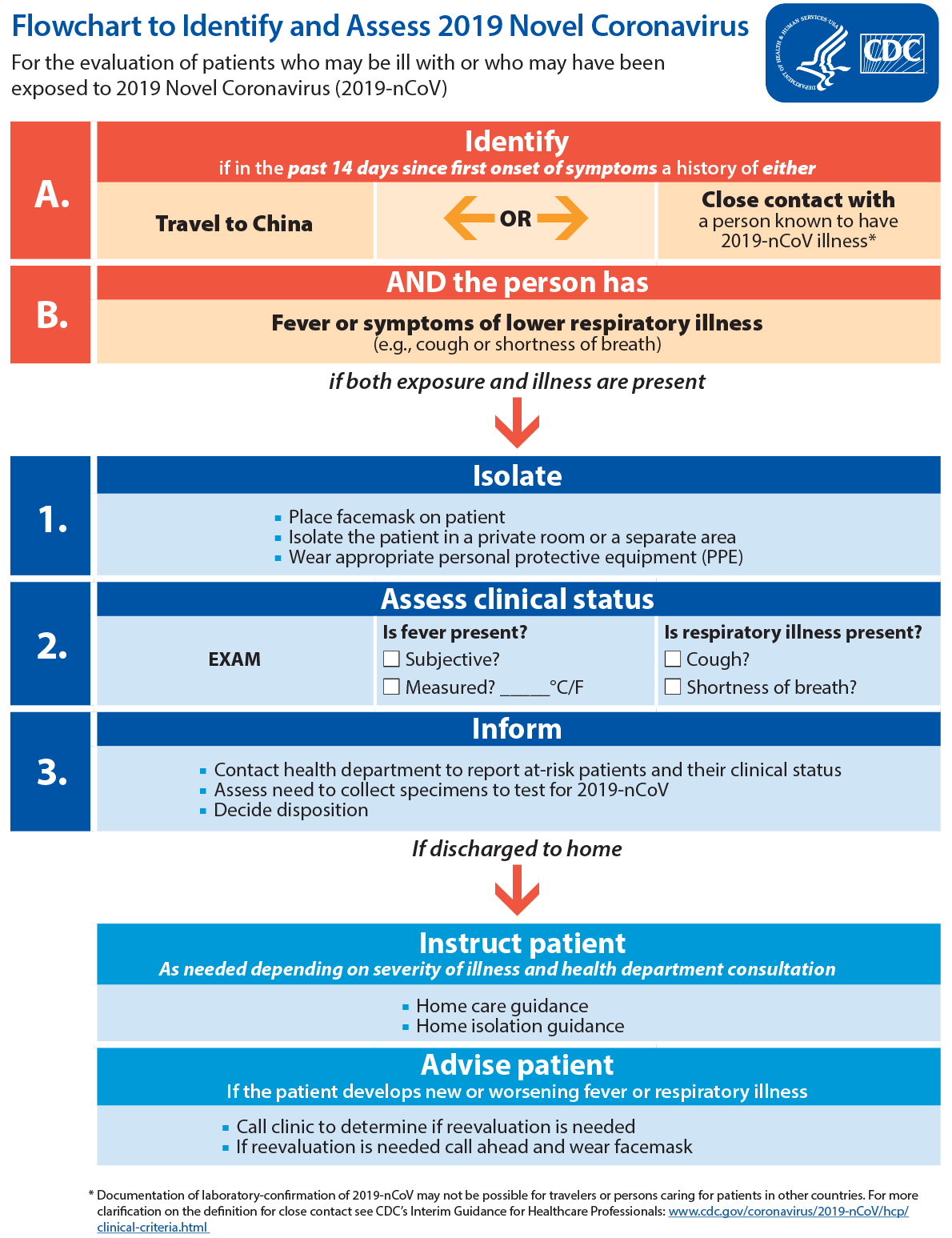
Text Equivalent
For the evaluation of patients who may be ill with or who may have been exposed to 2019 Novel Coronavirus (2019-nCoV)
- Identify if in the past 14 days since first onset of symptoms a history of either travel to china or close contact with a person known to have 2019-nCoV illness*
- AND the person has fever or symptoms of lower respiratory illness (e.g., cough or shortness of breath)
if both exposure and illness are present
- Isolate
- Place facemask on patient
- Isolate the patient in a private room or a separate area
- Wear appropriate personal protective equipment (PPE)
- Assess clinical status.
EXAM
Is fever present?
Subjective?
Measured? _____°C/F
Is respiratory illness present?
Cough?
Shortness of breath?
- Inform
- Contact health department to report at-risk patients and their clinical status
- Assess need to collect specimens to test for 2019-nCoV
- Decide disposition
If discharged to home
Instruct patient as needed depending on severity of illness and health department consultation
- Home care guidance
- Home isolation guidance
Advise patient if the patient develops new or worsening fever or respiratory illness
- Call clinic to determine if reevaluation is needed
- If reevaluation is needed call ahead and wear facemask
* Documentation of laboratory-confirmation of 2019-nCoV may not be possible for travelers or persons caring for patients in other countries. For more clarification on the definition for close contact see CDC’s Interim Guidance for Healthcare Professionals.
Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with 2019 Novel Coronavirus (2019-nCoV)
February 11th, 2020https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html
Background
CDC is closely monitoring an outbreak of respiratory illness caused by a novel (new) coronavirus (named by the World Health Organization as “2019-nCoV”) that was first detected in Wuhan, Hubei Province, China and which continues to expand. Chinese health officials have reported tens of thousands of infections with 2019-nCoV in China, with the virus spreading from person-to-person in many parts of that country. Transmission to healthcare personnel (HCP) in China has also been reported in the media; however, there is not yet detailed information about those transmission events. Infections with 2019-nCoV, most of them associated with travel from Wuhan, are also being reported from a growing number of international locations, including the United States. The first confirmed instance of person-to-person spread of 2019-nCoV in the United States was reported on January 30, 2020.
Much is unknown about 2019-nCoV. Current knowledge is largely based on what is known about similar coronaviruses. Coronaviruses are a large family of viruses that are common in humans and in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with SARS-CoV, MERS-CoV, and likely now with 2019-nCoV.
Early reports suggest spread from person-to-person most likely happens during close exposure to a person infected with 2019-nCoV. Person-to-person spread may occur similar to other coronaviruses, mainly via respiratory droplets produced when an infected person coughs. These droplets can land in the mouths, noses, or eyes of people who are nearby or possibly be inhaled into the lungs. Currently, the extent to which touching a surface or object that has the virus on it and then touching their own mouth, nose, or possibly their eyes, contributes to transmission is unclear.
Purpose
This interim guidance is intended to assist with assessment of risk, monitoring, and work restriction decisions for HCP with potential exposure to 2019-nCoV. For guidance on assessment and management of exposure risk in non-healthcare settings, refer to the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings. The guidance for non-healthcare settings can also be used to identify the movement, public activity and travel restrictions that apply to the HCP included here.
Because of their often extensive and close contact with vulnerable individuals in healthcare settings, a conservative approach to HCP monitoring and restriction from work was taken to quickly identify early symptoms and prevent transmission from potentially contagious HCP to patients, HCP, and others visiting or working in a healthcare setting. For this reason, the signs and symptoms* described in this guidance are more inclusive than those described when assessing exposures for individuals not working in healthcare. Healthcare facilities should have a low threshold for evaluating symptoms and testing symptomatic HCP, particularly those who fall into the high- and medium-risk categories described in this guidance.
This guidance is based on the currently limited information available about 2019-nCoV. Based on these uncertainties, the recommendations regarding which HCP are restricted from work may not prevent all transmission or anticipate every potential scenario, and will change if indicated by new information.
Healthcare facilities, in consultation with public health authorities should use clinical judgement as well as the principles outlined in this guidance to assign risk and determine need for work restrictions. CDC remains available for further consultation by calling the Emergency Operations Center at 770-488-7100. This cautious approach will be refined and updated as more information becomes available and as response needs change in the United States.
Other Resources
For guidance on risk assessment and public health management of persons not working in a U.S. healthcare setting refer to: Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings.
For infection prevention and control guidance for healthcare settings caring for Persons with Known or Under Investigation (PUI) for 2019 Novel Coronavirus (2019-nCoV), refer to the Interim Infection Prevention and Control Recommendations for Patients with Known or Persons Under Investigation for 2019 Novel Coronavirus (2019-nCoV) in a Healthcare Setting.
I. Definitions Used in this Guidance
Self-monitoring means HCP should monitor themselves for fever by taking their temperature twice a day and remain alert for respiratory symptoms (e.g., cough, shortness of breath, sore throat)*. Anyone on self-monitoring should be provided a plan for whom to contact if they develop fever or respiratory symptoms during the self-monitoring period to determine whether medical evaluation is needed.
Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat)*. For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. The mode of communication can be determined by the state or local public health authority and may include telephone calls or any electronic or internet-based means of communication.
For HCP, active monitoring can be delegated by the health department to the HCP’s healthcare facility occupational health or infection control program, if both the health department and the facility are in agreement. Note, inter-jurisdictional coordination will be needed if HCP live in a different local health jurisdiction than where the healthcare facility is located.
Self-Monitoring with delegated supervision in a healthcare setting means HCP perform self-monitoring with oversight by their healthcare facility’s occupational health or infection control program in coordination with the health department of jurisdiction, if both the health department and the facility are in agreement. Occupational health or infection control personnel should establish points of contact between the organization, the self-monitoring personnel, and the local or state health departments of authority in the location where self-monitoring personnel will be during the self-monitoring period. This communication should result in agreement on a plan for medical evaluation of personnel who develop fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat)* during the self-monitoring period. The plan should include instructions for notifying occupational health and the local public health authority, and transportation arrangements to a designated hospital, if medically necessary, with advance notice if fever or respiratory symptoms occur. The supervising organization should remain in contact with HCP through the self-monitoring period to oversee self-monitoring activities. Note, inter-jurisdictional coordination will be needed if HCP live in a different local health jurisdiction than where the healthcare facility is located.
Close contact for healthcare exposures is defined as follows: a) being within approximately 6 feet (2 meters), of a person with 2019-nCoV infection for a prolonged period of time (such as caring for or visiting the patient; or sitting within 6 feet of the patient in a healthcare waiting area or room); or b) having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand).
Data to inform the definition of close contact are limited. Considerations when assessing close contact include the duration of exposure (e.g., longer exposure time likely increases exposure risk), clinical symptoms of the patient (e.g., coughing likely increases exposure risk) and whether the patient was wearing a facemask (which can efficiently block respiratory secretions from contaminating others and the environment). It is not possible to define the duration of time that constitutes a prolonged exposure. However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1 to 2 minutes) exposure as prolonged.
Currently brief interactions are considered to be less likely to result in transmission; however, as described above, this is dependent on the clinical symptoms of the patient and type of interaction (e.g., did the patient cough directly into the face of the HCP). Information about this will be updated as more information becomes available. Risk stratification can be made in consultation with public health authorities. Examples of brief interactions include: briefly entering the patient room without having direct contact with the patient or their secretions/excretions, brief conversation at a triage desk with a patient who was not wearing a facemask. See Table 1 for more detailed information.
Healthcare Personnel: For the purposes of this document HCP refers to refers to all paid and unpaid persons serving in healthcare settings who have the potential for direct or indirect exposure to patients or infectious materials, including body substances; contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air. For this document, HCP does not include clinical laboratory personnel.
II. Defining Exposure Risk Category
While body fluids other than respiratory secretions have not been clearly implicated in transmission of 2019-nCoV, unprotected contact with other body fluids, including blood, stool, vomit, and urine, should also be considered as potentially putting HCP at risk of 2019-nCoV infection, until further data are available.
When assigning risk, factors to consider include: the duration of exposure (e.g., longer exposure time likely increases exposure risk), clinical symptoms of the patient (e.g., coughing likely increases exposure risk), whether the patient was wearing a facemask (which can efficiently block respiratory secretions from contaminating others and the environment), whether an aerosol generating procedure was performed, and the type of PPE used by HCP. However, data on the risk of transmission of 2019-nCoV are currently incomplete and the precision of current risk assignment is limited. Table 1 describes possible scenarios that can be used to assist with risk assessment. These scenarios do not cover all potential exposure scenarios and should not replace an individual assessment of risk for the purpose of clinical decision making or individualized public health management. Any public health decisions that place restrictions on an individual’s or group’s movements or impose specific monitoring requirements should be based on an assessment of risk for the individual or group. Healthcare facilities, in consultation with public health authorities should use the concepts outlined in this guidance along with clinical judgement to assign risk and determine need for work restrictions.
For this guidance high-risk exposures refer to HCP who performed or were present in the room for procedures that generate aerosols or during which respiratory secretions are likely to be poorly controlled (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) on patients with 2019-nCoV when the healthcare providers’ eyes, nose, or mouth were not protected.
Medium-risk exposures generally include HCP who had prolonged close contact with patients with 2019-nCoV where HCP mucous membranes or hands were exposed to material potentially infectious with 2019-nCoV. These exposures could place the exposed HCP at risk of developing disease that is less than that described under high-risk.
Proper adherence to currently recommended infection control practices, including all recommended PPE, should protect HCP having prolonged close contact with patients infected with 2019-nCoV. However, HCP in this category are classified as having low-risk to account for any inconsistencies in use or adherence that could result in unrecognized exposures.
HCP with no direct patient contact and no entry into active patient management areas who adhere to routine safety precautions are not considered to have a risk of exposure to 2019-nCoV (i.e., they have no identifiable risk.)
Currently the guidance is intended to apply to HCP with potential exposure in a healthcare setting to patients with confirmed 2019-nCoV infection. However, HCP exposures will commonly involve a PUI who is awaiting testing. Implementation of the monitoring and work restrictions described in this guidance could be applied to HCP exposed to a PUI if test results for the PUI are not expected to return within 48 to 72 hours. A record of HCP exposed to the PUI should still be maintained and HCP should be encouraged to perform self- monitoring while awaiting test results. If the results will be delayed more than 72 hours or the patient is positive for 2019-nCoV then all monitoring and work restrictions described in this document should be followed.
Table 1: Epidemiologic Risk Classification1 for Asymptomatic Healthcare Personnel Following Exposure to Patients with 2019 Novel Coronavirus (2019-nCoV) Infection or their Secretions/Excretions in a Healthcare Setting, and their Associated Monitoring and Work Restriction Recommendations
The distinction between the high- and medium-risk exposures in this document is somewhat artificial as they both place HCP at risk for developing infection; therefore the recommendations for active monitoring and work restrictions are the same for these exposures. However, these risk categories were created to align with risk categories described in the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings, which outlines criteria for quarantine and travel restrictions specific to high-risk exposures. Refer to that Interim Guidance for information about the movement, public activity and travel restrictions that apply to the HCP included here.
The highest risk exposure category that applies should be used to guide monitoring and work restrictions.
| Epidemiologic risk factors | Exposure category | Recommended Monitoring for 2019-nCoV (until 14 days after last potential exposure) | Work Restrictions for Asymptomatic HCP |
|---|---|---|---|
| A. HCP (with unprotected eyes, nose, or mouth) who perform or are present in the room for a procedure likely to generate higher concentrations of respiratory secretions or aerosols (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) | High | Active | Exclude from work for 14 days after last exposure |
| B. HCP who perform or are present in the room for a procedure likely to generate higher concentrations of respiratory secretions or aerosols (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) and not using a gown and gloves.Note: If the HCP’s eyes, nose, or mouth were also unprotected they would fall into the high-risk category above. | Medium | Active | Exclude from work for 14 days after last exposure |
| C. HCP (with unprotected eyes, nose, or mouth) who have prolonged close contact with a patient who was not wearing a facemaskNote: A respirator confers a higher level of protection than a facemask. However, they are group together in this scenario because (even if a respirator or facemask was worn) the eyes remain uncovered while having prolonged close contact with a patient who was not wearing a facemask. | Medium | Active | Exclude from work for 14 days after last exposure |
| D. HCP (with unprotected eye, nose, and mouth) who have prolonged close contact with a patient who was wearing a facemask | Medium | Active | Exclude from work for 14 days after last exposure |
| E. HCP (not wearing gloves) who have direct contact with the secretions/excretions of a patient and the HCP failed to perform immediate hand hygieneNote: If the HCP performed hand hygiene immediately after contact, this would be considered low risk. | Medium | Active | Exclude from work for 14 days after last exposure |
| F. HCP wearing a facemask or respirator only who have prolonged close contact with a patient who was wearing a facemaskNote: A respirator confers a higher level of protection than a facemask. However, they are grouped together in this scenario and classified as low-risk because the patient was wearing a facemask for source control. | Low | Self with delegated supervision | None |
| G. HCP using all recommended PPE (i.e., a respirator, eye protection, gloves and a gown) while caring for or having contact with the secretions/excretions of a patient | Low | Self with delegated supervision | None |
| H. HCP (not using all recommended PPE) who have brief interactions with a or patient regardless of whether patient was wearing a facemask (e.g., brief conversation at a triage desk; briefly entering a patient room but not having direct contact with the patient or their secretions/excretions; entering the patient room immediately after they have been discharged) | Low | Self with delegated supervision | None |
| I. HCP who walk by a patient or who have no direct contact with the patient or their secretions/excretions and no entry into the patient room | No identifiable risk | None | None |
HCP=healthcare personnel; PPE=personal protective equipment
1 The distinction between the high- and medium-risk exposures in this document is somewhat artificial as they both place HCP at risk for developing infection and the recommendations for active monitoring and work restrictions are the same for these exposures. However, these risk categories were created to align with risk categories described in the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings, which outlines criteria for quarantine and travel restrictions specific to high-risk exposures. Refer to that Interim Guidance for information about the movement, public activity and travel restrictions that apply to the HCP included here.
III. Recommendations for Monitoring Based on 2019-nCoV Exposure Risk
-
High- and Medium-risk Exposure Category
HCP in the high- or medium-risk category should undergo active monitoring, including restriction from work in any healthcare setting until 14 days after their last exposure. If they develop any fever (measured temperature >100.0oF or subjective fever) OR respiratory symptoms consistent with 2019-nCoV infection (e.g., cough, shortness of breath, sore throat)* they should immediately self-isolate (separate themselves from others) and notify their local or state public health authority and healthcare facility promptly so that they can coordinate consultation and referral to a healthcare provider for further evaluation.
-
Low-risk Exposure Category
HCP in the low-risk category should perform self-monitoring with delegated supervision until 14 days after the last potential exposure. Asymptomatic HCP in this category are not restricted from work. They should check their temperature twice daily and remain alert for respiratory symptoms consistent with 2019-nCoV infection infection (e.g., cough, shortness of breath, sore throat)*. They should ensure they are afebrile and asymptomatic before leaving home and reporting for work. If they do not have fever or respiratory symptoms they may report to work. They should have their temperature retaken and symptoms assessed by the healthcare facility each day before starting work. On days they are not working they are not required to report unless they develop symptoms. If they develop fever (measured temperature > 100.0 oF or subjective fever) OR respiratory symptoms they should immediately self-isolate (separate themselves from others) and notify their local or state public health authority or healthcare facility promptly so that they can coordinate consultation and referral to a healthcare provider for further evaluation.
-
No Identifiable risk Exposure Category
HCP in the no identifiable risk category do not require monitoring or restriction from work.
-
Community or travel-associated exposures
HCP with potential exposures to 2019-nCoV in community settings, should have their exposure risk assessed according CDC guidance. HCP who fall into the high- or medium- risk category described there should undergo monitoring as defined by their local or state public health authority and be excluded from work in a healthcare setting until 14 days after their exposure. HCP who develop signs or symptoms compatible with 2019-nCoV should contact their established point of contact (public health authorities or their facility’s occupational health program) for medical evaluation prior to returning to work.
*Fever is either measured temperature >100.0oF or subjective fever. Note that fever may be intermittent or may not be present in some patients, such as those who are elderly, immunosuppressed, or taking certain medications (e.g., NSAIDs). Clinical judgement should be used to guide testing of patients in such situations. Respiratory symptoms consistent with 2019-nCoV infection are cough, shortness of breath, and sore throat. Medical evaluation may be recommended for lower temperatures (<100.0oF) or other symptoms (e.g., muscle aches, nausea, vomiting, diarrhea, abdominal pain headache, runny nose, fatigue) based on assessment by public health authorities.
CDC: Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for 2019-nCoV in the United States
February 11th, 2020https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html
Background
Emergency medical services (EMS) play a vital role in responding to requests for assistance, triaging patients, and providing emergency medical treatment and transport for ill persons. However, unlike patient care in the controlled environment of a healthcare facility, care and transports by EMS present unique challenges because of the nature of the setting, enclosed space during transport, frequent need for rapid medical decision-making, interventions with limited information, and a varying range of patient acuity and jurisdictional healthcare resources.
When preparing for and responding to patients with confirmed or possible 2019-Novel Coronavirus (2019-nCov) infection, close coordination and effective communications are important among 911 Public Safety Answering Points (PSAPs)— commonly known as 911 call centers, the EMS system, healthcare facilities, and the public health system. Each PSAP and EMS system should seek the involvement of an EMS medical director to provide appropriate medical oversight. For the purposes of this guidance, “EMS clinician” means prehospital EMS and medical first responders. When 2019-nCoV is suspected in a patient needing emergency transport, prehospital care providers and healthcare facilities should be notified in advance that they may be caring for, transporting, or receiving a patient who may have 2019-nCoV infection.
Updated information about 2019-nCov may be accessed at https://www.cdc.gov/coronavirus/2019-ncov/index.html. Infection prevention and control recommendations can be found here: https://www.cdc.gov/coronavirus/2019-nCoV/hcp/infection-control.html. Additional information for healthcare personnel can be found at https://www.cdc.gov/coronavirus/2019-nCoV/guidance-hcp.html.
Case Definition for 2019-nCoV
CDC’s most current case definition for a person under investigation (PUI) for 2019-nCov may be accessed at https://www.cdc.gov/coronavirus/2019-nCoV/clinical-criteria.html.
Recommendations for 911 PSAPs
Municipalities and local EMS authorities should coordinate with state and local public health, PSAPs, and other emergency call centers to determine need for modified caller queries about 2019-nCoV, outlined below.
Development of these modified caller queries should be closely coordinated with an EMS medical director and informed by local, state, and federal public health authorities, including the city or county health department(s), state health department(s), and CDC.
Modified Caller Queries
PSAPs or Emergency Medical Dispatch (EMD) centers (as appropriate) should question callers and determine the possibility that this call concerns a person who may have signs or symptoms and risk factors for 2019-nCoV. The query process should never supersede the provision of pre-arrival instructions to the caller when immediate lifesaving interventions (e.g., CPR or the Heimlich maneuver) are indicated. Patients in the United States who meet the appropriate criteria should be evaluated and transported as a PUI. Information on 2019-nCoV will be updated as the public health response proceeds. PSAPs and medical directors can access CDC’s PUI definitions here.
Information on a possible PUI should be communicated immediately to EMS clinicians before arrival on scene in order to allow use of appropriate personal protective equipment (PPE). PSAPs should utilize medical dispatch procedures that are coordinated with their EMS medical director and with the local or state public health department.
PSAPs and EMS units that respond to ill travelers at US international airports or other ports of entry to the United States (maritime ports or border crossings) should be in contact with the CDC quarantine station of jurisdiction for the port of entry (see: CDC Quarantine Station Contact List) for planning guidance. They should notify the quarantine station when responding to that location if a communicable disease is suspected in a traveler. CDC has provided job aids for this purpose to EMS units operating routinely at US ports of entry. The PSAP or EMS unit can also call CDC’s Emergency Operations Center at (770)488-7100 to be connected with the appropriate CDC quarantine station.
Recommendations for EMS Clinicians and Medical First Responders
EMS clinician practices should be based on the most up-to-date 2019-nCoV clinical recommendations and information from appropriate public health authorities and EMS medical direction.
State and local EMS authorities may direct EMS clinicians to modify their practices as described below.
Patient assessment
- If PSAP call takers advise that the patient is suspected of having 2019-nCoV, EMS clinicians should put on appropriate PPE before entering the scene. EMS clinicians should consider the signs, symptoms, and risk factors of 2019-nCoV (https://www.cdc.gov/coronavirus/2019-nCoV/clinical-criteria.html).
- If information about potential for 2019-nCoV has not been provided by the PSAP, EMS clinicians should exercise appropriate precautions when responding to any patient with signs or symptoms of a respiratory infection. Initial assessment should begin from a distance of at least 6 feet from the patient, if possible. Patient contact should be minimized to the extent possible until a facemask is on the patient. If 2019-nCoV infection is suspected, all PPE as described below should be used. If 2019-nCoV infection is not suspected, EMS clinicians should follow standard procedures and use appropriate PPE for evaluating a patient with a potential respiratory infection.
- A facemask should be worn by the patient for source control. If a nasal cannula is in place, a facemask should be worn over the nasal cannula. Alternatively, an oxygen mask can be used if clinically indicated. If the patient requires intubation, see below for additional precautions for aerosol-generating procedures.
- During transport, limit the number of providers in the patient compartment to essential personnel to minimize possible exposures.
Recommended Personal Protective Equipment (PPE)
- EMS clinicians who will directly care for a patient with possible 2019-nCoV infection or who will be in the compartment with the patient should follow Standard, Contact, and Airborne Precautions, including the use of eye protection. Recommended PPE includes:
- A single pair of disposable patient examination gloves. Change gloves if they become torn or heavily contaminated,
- Disposable isolation gown,
- Respiratory protection (i.e., N-95 or higher-level respirator), and
- Eye protection (i.e., goggles or disposable face shield that fully covers the front and sides of the face).
- Drivers, if they provide direct patient care (e.g., moving patients onto stretchers), should wear all recommended PPE . After completing patient care and before entering an isolated driver’s compartment, the driver should remove and dispose of PPE and perform hand hygiene to avoid soiling the compartment.
- If the transport vehicle does not have an isolated driver’s compartment, the driver should remove the face shield or goggles, gown and gloves and perform hand hygiene. A respirator should continue to be used during transport.
- All personnel should avoid touching their face while working.
- On arrival, after the patient is released to the facility, EMS clinicians should remove and discard PPE and perform hand hygiene. Used PPE should be discarded in accordance with routine procedures.
- Other required aspects of Standard Precautions (e.g., injection safety, hand hygiene) are not emphasized in this document but can be found in the guideline titled Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.
Precautions for Aerosol-Generating Procedures
- If possible, consult with medical control before performing aerosol-generating procedures for specific guidance.
- In addition to the PPE described above, EMS clinicians should exercise caution if an aerosol-generating procedure (e.g., bag valve mask (BVM) ventilation, oropharyngeal suctioning, endotracheal intubation, nebulizer treatment, continuous positive airway pressure (CPAP), bi-phasic positive airway pressure (biPAP), or resuscitation involving emergency intubation or cardiopulmonary resuscitation (CPR)) is necessary.
- BVMs, and other ventilatory equipment, should be equipped with HEPA filtration to filter expired air.
- EMS organizations should consult their ventilator equipment manufacturer to confirm appropriate filtration capability and the effect of filtration on positive-pressure ventilation.
- If possible, the rear doors of the transport vehicle should be opened and the HVAC system should be activated during aerosol-generating procedures. This should be done away from pedestrian traffic.
EMS Transport of a PUI or Patient with Confirmed 2019-nCoV to a Healthcare Facility (including interfacility transport)
If a patient with an exposure history and signs and symptoms suggestive of 2019-nCoV infection requires transport to a healthcare facility for further evaluation and management (subject to EMS medical direction), the following actions should occur during transport:
- EMS clinicians should notify the receiving healthcare facility that the patient has an exposure history and signs and symptoms suggestive of 2019-nCoV infection so that appropriate infection control precautions may be taken prior to patient arrival.
- Keep the patient separated from other people as much as possible.
- Family members and other contacts of patients with possible 2019-nCoV infection should not ride in the transport vehicle, if possible. If riding in the transport vehicle, they should wear a facemask.
- Isolate the ambulance driver from the patient compartment and keep pass-through doors and windows tightly shut.
- When possible, use vehicles that have isolated driver and patient compartments that can provide separate ventilation to each area.
- Close the door/window between these compartments before bringing the patient on board.
- During transport, vehicle ventilation in both compartments should be on non-recirculated mode to maximize air changes that reduce potentially infectious particles in the vehicle.
- If the vehicle has a rear exhaust fan, use it to draw air away from the cab, toward the patient-care area, and out the back end of the vehicle.
- Some vehicles are equipped with a supplemental recirculating ventilation unit that passes air through HEPA filters before returning it to the vehicle. Such a unit can be used to increase the number of air changes per hour (ACH) (https://www.cdc.gov/niosh/hhe/reports/pdfs/1995-0031-2601.pdfpdf icon).
- If a vehicle without an isolated driver compartment and ventilation must be used, open the outside air vents in the driver area and turn on the rear exhaust ventilation fans to the highest setting. This will create a negative pressure gradient in the patient area.
- Follow routine procedures for a transfer of the patient to the receiving healthcare facility (e.g., wheel the patient directly into an Airborne Infection Isolation Room).
Documentation of patient care
- Documentation of patient care should be done after EMS clinicians have completed transport, removed their PPE, and performed hand hygiene.
- Any written documentation should match the verbal communication given to the emergency department providers at the time patient care was transferred.
- EMS documentation should include a listing of EMS clinicians and public safety providers involved in the response and level of contact with the patient (for example, no contact with patient, provided direct patient care). This documentation may need to be shared with local public health authorities.
Cleaning EMS Transport Vehicles after Transporting a PUI or Patient with Confirmed 2019-nCoV
The following are general guidelines for cleaning or maintaining EMS transport vehicles and equipment after transporting a PUI:
- After transporting the patient, leave the rear doors of the transport vehicle open to allow for sufficient air changes to remove potentially infectious particles.
- The time to complete transfer of the patient to the receiving facility and complete all documentation should provide sufficient air changes.
- When cleaning the vehicle, EMS clinicians should wear a disposable gown and gloves. A face shield or facemask and goggles should also be worn if splashes or sprays during cleaning are anticipated.
- Ensure that environmental cleaning and disinfection procedures are followed consistently and correctly, to include the provision of adequate ventilation when chemicals are in use. Doors should remain open when cleaning the vehicle.
- Routine cleaning and disinfection procedures (e.g., using cleaners and water to pre-clean surfaces prior to applying an EPA-registered, hospital-grade disinfectant to frequently touched surfaces or objects for appropriate contact times as indicated on the product’s label) are appropriate for 2019-nCoV in healthcare settings, including those patient-care areas in which aerosol-generating procedures are performed.
- Products with EPA-approved emerging viral pathogens claims are recommended for use against 2019-nCoV. These products can be identified by the following claim:
- “[Product name] has demonstrated effectiveness against viruses similar to 2019-nCoV on hard non-porous surfaces. Therefore, this product can be used against 2019-nCoV when used in accordance with the directions for use against [name of supporting virus] on hard, non-porous surfaces.”
- This claim or a similar claim, will be made only through the following communications outlets: technical literature distributed exclusively to health care facilities, physicians, nurses and public health officials, “1-800” consumer information services, social media sites and company websites (non-label related). Specific claims for “2019-nCoV” will not appear on the product or master label.
- See additional information about EPA-approved emerging viral pathogens claimsexternal icon.
- If there are no available EPA-registered products that have an approved emerging viral pathogen claim, products with label claims against human coronaviruses should be used according to label instructions.
- Clean and disinfect the vehicle in accordance with standard operating procedures. All surfaces that may have come in contact with the patient or materials contaminated during patient care (e.g., stretcher, rails, control panels, floors, walls, work surfaces) should be thoroughly cleaned and disinfected using an EPA-registered hospital grade disinfectant in accordance with the product label.
- Clean and disinfect reusable patient-care equipment before use on another patient, according to manufacturer’s instructions.
- Follow standard operating procedures for the containment and disposal of used PPE and regulated medical waste.
- Follow standard operating procedures for containing and laundering used linen. Avoid shaking the linen.
Follow-up and/or Reporting Measures by EMS Clinicians After Caring for a PUI or Patient with Confirmed 2019-nCoV
EMS clinicians should be aware of the follow-up and/or reporting measures they should take after caring for a PUI or patient with confirmed 2019-nCoV:
- State or local public health authorities should be notified about the patient so appropriate follow-up monitoring can occur.
- EMS agencies should develop policies for assessing exposure risk and management of EMS personnel potentially exposed to 2019-nCoV in coordination with state or local public health authorities. Decisions for monitoring, excluding from work, or other public health actions for HCP with potential exposure to 2019-nCoV should be made in consultation with state or local public health authorities. Refer to the Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with 2019 Novel Coronavirus (2019-nCoV) for additional information.
- EMS agencies should develop sick-leave policies for EMS personnel that are nonpunitive, flexible, and consistent with public health guidance. Ensure all EMS personnel, including staff who are not directly employed by the healthcare facility but provide essential daily services, are aware of the sick-leave policies.
- EMS personnel who have been exposed to a patient with suspected or confirmed 2019-nCoV should notify their chain of command to ensure appropriate follow-up.
- Any unprotected exposure (e.g., not wearing recommended PPE) should be reported to occupational health services, a supervisor, or a designated infection control officer for evaluation.
- EMS clinicians should be alert for fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). If symptoms develop, they should self-isolate and notify occupational health services and/or their public health authority to arrange for appropriate evaluation.
EMS Employer Responsibilities
The responsibilities described in this section are not specific for the care and transport of PUIs or patients with confirmed 2019-nCoV. However, this interim guidance presents an opportunity to assess current practices and verify that training and procedures are up-to-date.
- EMS units should have infection control policies and procedures in place, including describing a recommended sequence for safely donning and doffing PPE.
- Provide all EMS clinicians with job- or task-specific education and training on preventing transmission of infectious agents, including refresher training.
- Ensure that EMS clinicians are educated, trained, and have practiced the appropriate use of PPE prior to caring for a patient, including attention to correct use of PPE and prevention of contamination of clothing, skin, and environment during the process of removing such equipment.
- Ensure EMS clinicians are medically cleared, trained, and fit tested for respiratory protection device use (e.g., N95 filtering facepiece respirators), or medically cleared and trained in the use of an alternative respiratory protection device (e.g., Powered Air-Purifying Respirator, PAPR) whenever respirators are required. OSHA has a number of respiratory training videosexternal icon.
- EMS units should have an adequate supply of PPE.
- Ensure an adequate supply of or access to EPA-registered hospital grade disinfectants (see above for more information) for adequate decontamination of EMS transport vehicles and their contents.
- Ensure that EMS clinicians and biohazard cleaners contracted by the EMS employer tasked to the decontamination process are educated, trained, and have practiced the process according to the manufacturer’s recommendations or the EMS agency’s standard operating procedures.
Additional Resources
The EMS Infectious Disease Playbook, published by the Office of the Assistant Secretary for Preparedness and Response’s Technical Resources, Assistance Center, Information Exchange (TRACIE) is a resource available to planners at https://www.ems.gov/pdf/ASPR-EMS-Infectious-Disease-Playbook-June-2017.pdfpdf iconexternal icon
CDC: Healthcare Supply of Personal Protective Equipment
February 11th, 2020https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html
CDC continues to monitor the 2019-nCoV situation in the United States and around the world. CDC has taken early and aggressive actions to prevent the spread of 2019-nCoV in the United States, through a combination of proven public health actions. At the same time, CDC is preparing for the possibility that the 2019-nCoV situation in the US could become more serious, with sustained community transmission, and is taking steps to make sure there are enough supplies and appropriate guidance to prevent spread of disease, especially among healthcare personnel caring for patients with 2019-nCoV.
Healthcare personnel can protect themselves when caring for patients by adhering to infection prevention and control practices, which includes the appropriate use of engineering controls, administrative controls, and personal protective equipment (PPE). CDC has issued guidance recommending the use of PPE for healthcare personnel caring for patients with confirmed or possible 2019-nCoV infection. Employers and healthcare personnel are reminded that PPE is only one aspect of safe care of patients with 2019-nCoV. For the general public, CDC does not recommend the use of facemasks or respirators. CDC guidance is based on what we know about 2019-nCoV and what we know about similar coronaviruses, like SARS and MERS.
CDC also understands the importance of providing guidance that healthcare facilities can implement, given supplies of PPE available. CDC communicates regularly with healthcare industry partners, as well as PPE manufacturers and distributors, to assess availability of PPE. At this time, some partners are reporting higher than usual demand for select N95 respirators and facemasks. If information about market availability changes, updates will be posted on this page.
Based on the current 2019-nCoV situation and availability of PPE, CDC has specific recommendations, summarized below. As we learn more about 2019-nCoV and as the needs of the response or availability of PPE within U.S. healthcare facilities changes, we will update our guidance.
Who needs PPE:
Patients with confirmed or possible 2019-nCoV infection should wear a facemask when being evaluated medically.
Healthcare personnel should adhere to Standard, Contact, and Airborne Precautions, including the use of eye protection (e.g., goggles or a face shield) when caring for patients with 2019-nCoV infection. These precautions include the use of PPE, including NIOSH-approved N95 respirators, gowns, gloves, face shield/eye protection, etc. This includes, but is not limited to, surgical N95 respirators.
Who does not need PPE:
CDC does NOT currently recommend the general public use facemasks. Instead, CDC recommends following everyday preventive actions, such as washing your hands, covering your cough, and staying home when you are sick.
Manufacturers and Distributors:
Cases of 2019-nCoV are being reported in China as well as other countries. Given decreases in exports from select countries (e.g., China, India, Taiwan) and increases in demand due to the outbreak, manufacturers of select types of PPE are reporting increased volume of orders and challenges in meeting order demands. Plans to surge manufacturing globally are underway.
Strategies for Optimizing Supply of N95 Respirators
CDC offers strategies for healthcare settings on how to optimize supplies of N95 respirators in the face of decreasing supply. These strategies are organized using the occupational health and safety hierarchy of controls approach.
Frequently Asked Questions About Respirators and Their Use
CDC answers frequently asked questions about respirators and their use for healthcare personnel and the general public.
Rohingya refugee boat sinks: Most of the victims were woman and children, fleeing the overcrowded refugee camps for Rohingya Muslims in Bangladesh.
February 11th, 2020Information for Healthcare Professionals on 2019-nCoV
February 11th, 2020
https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html
Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with 2019 Novel Coronavirus (2019-nCoV)
https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html
Interim Guidance for Emergency Medical Services (EMS) Systems and 911 Public Safety Answering Points (PSAPs) for 2019-nCoV in the United States
https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-for-ems.html
Healthcare Supply of Personal Protective Equipment
https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html




