A fatal case of MERS-CoV in a pregnant female
January 2nd, 2016
Malik A, El Masry KM, Ravi M, Sayed F. Middle East respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg Infect Dis. 2016 Mar [date cited]. http://dx.doi.org/10.3201/eid2203.151049
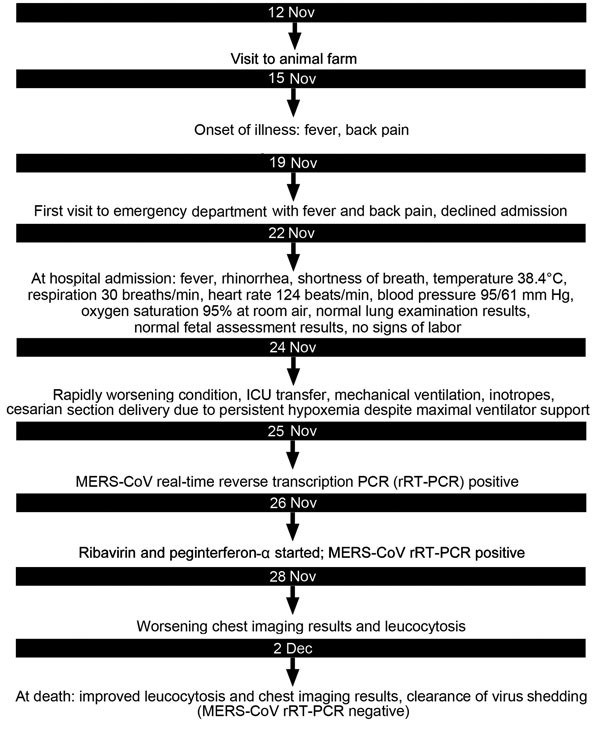
“…..On November 19, 2013, a 32-year-old woman residing in Abu-Dhabi, United Arab Emirates, sought medical care for fever and back pain of 4 days’ duration. The woman, a school teacher from Jordan, was 32 weeks pregnant; she reported 3 earlier pregnancies (2 live births) and no concurrent conditions. Emergency department (ED) and obstetric physicians suspected urinary tract infection. The patient declined admission but returned to the ED on November 22 with worsening fever, cough, and shortness of breath. She denied recent travel, sick contacts, or animal exposure within the previous 2 weeks. Lung examination results were within normal limits; the patient had no signs of active labor or fetal distress. Chest computed tomography scan revealed bilateral consolidation; pulmonary embolus was not seen.
The patient was admitted to the medical unit with suspected community-acquired pneumonia. Ceftriaxone and azithromycin were initiated. Her condition deteriorated, and on November 23, she was transferred to the intensive care unit (ICU) because of respiratory failure and hypotension. On November 24, acute respiratory distress syndrome developed, requiring respiratory and hemodynamic support. Empiric oseltamivir and vancomycin were added to the treatment regimen. Later that day, the baby was delivered by caesarean section because the patient was persistently hypoxemic while on maximal ventilator support. Transient improvement in oxygenation was noted after the delivery. The newborn, who was noted to be healthy and had Apgar scores of 6 and 8 at 1 and 5 minutes, respectively, had no contact with the mother after birth.
Nasopharyngeal aspirate samples were tested for influenza A(H1N1)pdm09 virus and MERS-CoV by real-time reverse transcription PCR (2), and multiple other laboratory and culture tests were conducted (Table(http://wwwnc.cdc.gov/eid/article/22/3/15-1049-t1)). Most yielded negative results, but on November 25, the regional laboratory reported the MERS-CoV real-time reverse transcription PCR results were positive. Laboratory testing was done by qualitative assay, using the 2012 novel human CoV (human coronavirus–Erasmus Medical Center). The assay, performed according to a previously described method (3), contains reagents and enzyme for specific amplification of the region upstream of the envelope gene in the CoV genome.
On November 26, oral ribavirin (400 mg and 600 mg morning and evening, respectively) and subcutaneous peginterferon-α (180 µg 1×/wk) were initiated. On November 27, ribavirin was increased to 1,200 mg every 8 hours, and meropenem was begun. Septic shock developed in the patient, requiring maximal vasopressors and ventilator support. Despite intensive support, the patient’s condition continued to deteriorate; she died on December 2. Cultures of blood, tracheal aspirate, and urine obtained on the day of death showed no growth; a chest radiograph revealed improvement in pulmonary edema and consolidation.