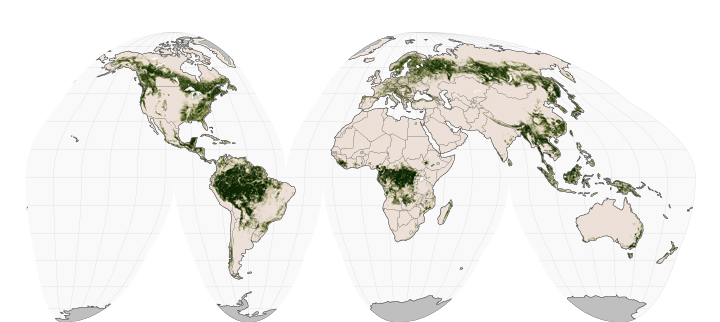
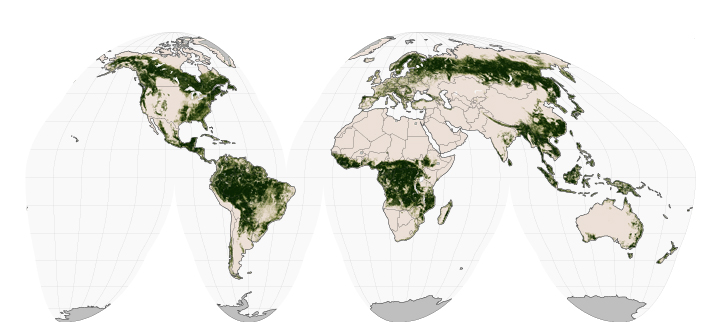
Archive for November, 2015
CHIKV is a significant cause of CNS disease
Monday, November 30th, 2015** Between September 2005 and June 2006, 57 patients were diagnosed with CHIKV-associated CNS disease, including 24 with CHIKV-associated encephalitis, the latter corresponding to a CIR (cumulative incidence rate ) of 8.6 per 100,000 persons.
** Patients with encephalitis were observed at both extremes of age categories.
** 187/100 000 (cumulative incidence rate ) in patients under 1 year of age
** 37/100 000 (cumulative incidence rate ) in patients over 65 years of age
** The case-fatality rate of CHIKV-associated encephalitis was 16.6%
** The proportion of children discharged with persistent disabilities estimated between 30% and 45%.
** Beyond the neonatal period, the clinical presentation and outcomes were less severe in infants than in adults.
Deluge in the Amargosa and Death Valleys, November 4, 2015
Sunday, November 29th, 2015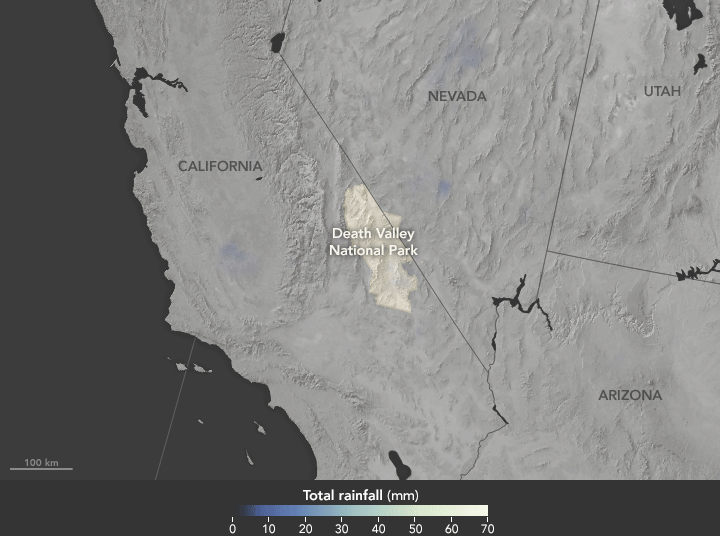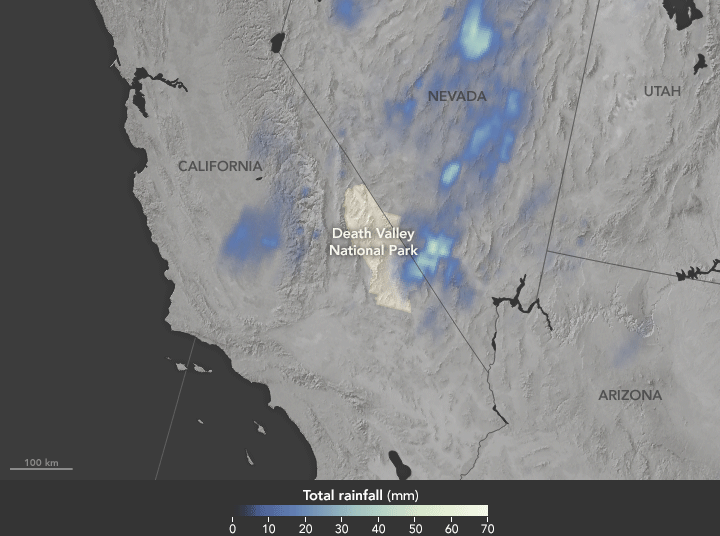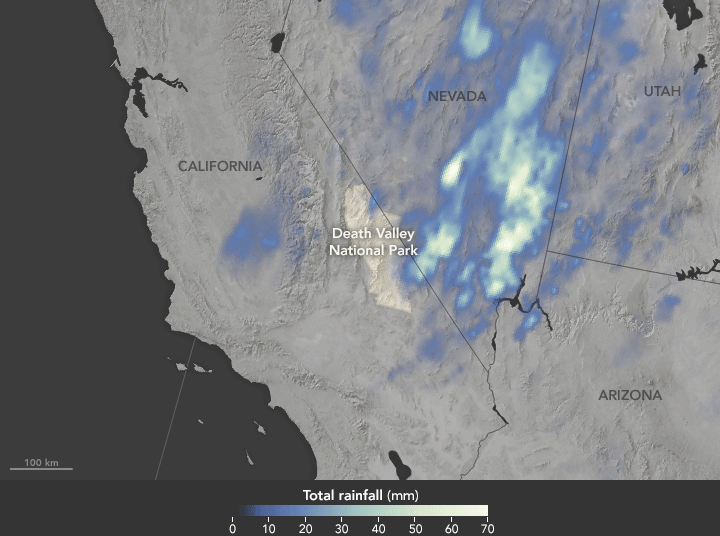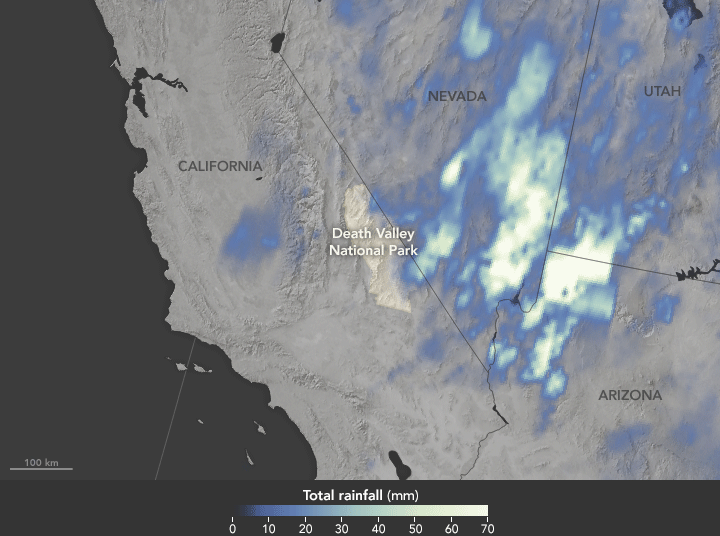
The animation above shows satellite-based estimates of rainfall from a particularly large storm that passed over the area on October 18. The rainfall data are from the Integrated Multi-Satellite Retrievals for GPM (IMERG), a product of the Global Precipitation Measurement mission. Green-white colors represent the largest rainfall totals, which in some areas reached upward of 70 millimeters (3 inches) during the 24-hour period displayed in the animation. These regional, remotely-sensed estimates may differ from the totals measured by ground-based weather stations.
UNICEF: The number of adolescent deaths from AIDS has tripled over the last 15 years
Sunday, November 29th, 2015The data reveal that currently among adolescents (15-19):
- 26 new infections occur every hour; and
- About half of those living with HIV are in just six countries:
- South Africa
- Nigeria
- Kenya
- India
- Mozambique
- Tanzania.
UNICEF’s Statistical Update on Children, Adolescents and AIDS report can be downloaded here: www.childrenandaids.org
New Chikungunya Research: Encephalitis had been diagnosed in 8.6 per 100,000 people in Reunion (2005-6)
Saturday, November 28th, 2015** PAHO: In the western hemisphere, more than 600,000 cases have been reported so far this year, with 76 deaths
** In 2013 and 2014 more than 1.1 million cases were reported and it killed 194 people.
FEMA Daily Operations Briefing for Friday, November 27, 2015
Friday, November 27th, 2015
Significant Events: None
Tropical Activity:
Atlantic: Tropical cyclone activity is not expected during the next 48 hours
Eastern Pacific: Hurricane Sandra (Cat 2)
Central Pacific: Tropical cyclone activity not expected through Saturday evening
Western Pacific: Tropical Storm 03F
Significant Weather:
Snow – the Cascades, Central Plains, Central Rockies & Upper Mississippi Valley
Freezing rain – Central/Southern Plains
Flash Flooding – Southern/Central Plains
Rain – Southern Plains to the Middle Mississippi Valley and into the Great Lakes
Red Flag Warnings: None
Critical/Elevated Fire Weather Areas: NM & TX
Space weather: Past 24 hours – none, Next 24 hours – none
Declaration Activity:
Amendment No. 1 for American Samoa (FEMA-4192-DR)
Major Disaster Declaration (FEMA-4245-DR) for Texas
- FEMA Daily Ops Briefing 11-27-2015: http://content.govdelivery.com/attachments/USDHSFEMA/2015/11/27/file_attachments/453939/FEMA%2BDaily%2BOps%2BBriefing%2B11-27-2015.pdf
An experimental vaccine to protect against the mosquito-borne illness chikungunya is being tested in a Phase 2 trial
Friday, November 27th, 2015 This digitally-colorized transmission electron micrograph (TEM) depicts numerous chikungunya virus particles, which are composed of a central dense core that is surrounded by a viral envelope.
This digitally-colorized transmission electron micrograph (TEM) depicts numerous chikungunya virus particles, which are composed of a central dense core that is surrounded by a viral envelope.
Credit: CDC
NIH-Sponsored Clinical Trial of Chikungunya Vaccine Opens
An experimental vaccine to protect against the mosquito-borne illness chikungunya is being tested in a Phase 2 trial sponsored by the National Institutes of Health. Results from an initial trial of the vaccine, which was developed by scientists at the NIH National Institute of Allergy and Infectious Diseases (NIAID), were reported in 2014. In that study, all 25 vaccine recipients developed robust immune responses and no safety concerns were noted. The new trial is designed to enroll 400 healthy adult volunteers aged 18 to 60 years old at six sites in the Caribbean. It will continue to gather data on the candidate vaccine’s safety and ability to elicit immune responses, including antibodies.
###
References:
L-J Chang et al. Chikungunya virus-like particle vaccine elicits neutralizing antibodies in healthy adults in a phase I dose escalation clinical trial. The Lancet DOI: 10.1016/S0140-6736(14)61185-5 (2014).