Archive for February, 2016
A twin suicide bombing claimed by Islamic State killed 70 people in a Shi’ite district of Baghdad on Sunday
Monday, February 29th, 2016
** “…..the suicide bombers were riding motorcycles and blew themselves up in a crowded mobile phone market in Sadr City, wounding more than 100 people in addition to the dead….”
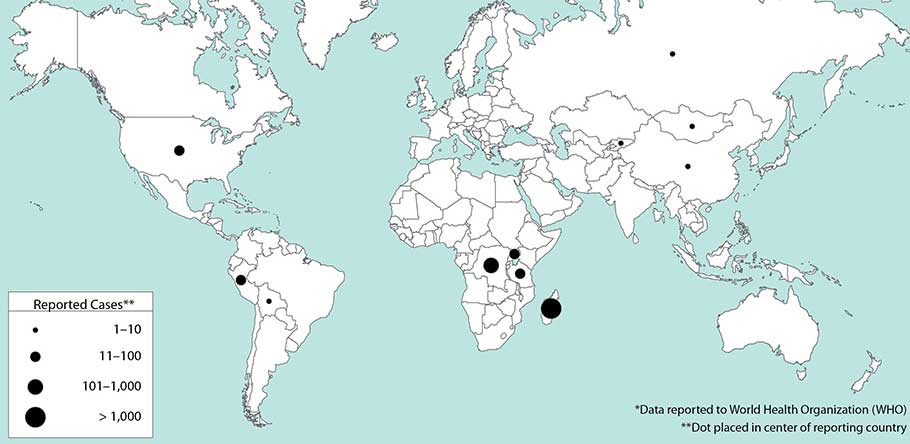
** Worldwide, between 1 January 2010 and 31 December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths.
Monday, February 29th, 2016
Weekly epidemiological record
26 FEBRUARY 2016, 91th YEAR
No 8, 2016, 91, 89–104
http://www.who.int/wer
India: A man fatally stabbed 14 members of his family, including seven children, early Sunday before hanging himself.
Sunday, February 28th, 2016Ebola: 84 Survivors reported musculoskeletal pain (70%), headache (48%), and ocular problems (14%).
Sunday, February 28th, 2016Scott JT, Sesay FR, Massaquoi TA, Idriss BR, Sahr F, Semple MG. Post-Ebola syndrome, Sierra Leone. Emerg Infect Dis. 2016 Apr [date cited]. http://dx.doi.org/10.32032/eid2204.151302
Table 5
Post-Ebola complaints other than headache, musculoskeletal pain, or ocular problems among 44 survivors, Sierra Leone
| Complaint | No. (%; 95% CI, %) |
|---|---|
| Cough | 5 (11; 4–25) |
| Abdominal pain | 4 (9; 3–22) |
| Chest pain | 4 (9; 3–22) |
| Itching | 4 (9; 3–22) |
| Insomnia | 3 (7; 1–19) |
| Fever | 3 (7; 1–19) |
| Loss of appetite | 3 (7; 1–19) |
| Labored speech | 2 (5; 1–15) |
| Epigastric pain | 2 (5; 1–15) |
| Rash | 2 (5; 1–5) |
| Other* | 1 (2; 0–12) |
*Weight loss, hiccups, increased appetite, chest pain, sneezing, diarrhea, vomiting, left sided weakness with facial nerve palsy, breathlessness, rash, dry flaky skin, earache, fever blister/cold sore, left scrotal swelling, nasal congestion, tremors.
Table 3
Musculoskeletal symptoms described by 31 patients with post-Ebola syndrome, Sierra Leone*
| Area of pain | Patient sex
|
Total | |
|---|---|---|---|
| M | F | ||
| Joints | |||
| Joint, unspecified | 5 | 9 | 14 |
| Knee, unspecified | 2 | 0 | 2 |
| Right knee joint | 0 | 1 | 1 |
| Shoulder joint
|
1
|
1
|
2
|
| Body | |||
| Generalized body | 4 | 4 | 8 |
| Upper back | 1 | 3 | 4 |
| Musculoskeletal, unspecified | 2 | 0 | 2 |
| Left thigh | 1 | 1 | 2 |
| Lower limb | 0 | 1 | 1 |
| Right thigh | 1 | 0 | 1 |
| Gluteal muscle | 1 | 0 | 1 |
*Values are no. patients. Some survivors reported >1 area of pain. The proportion of male and female survivors with musculoskeletal pain did not differ significantly (χ2, p = 0.7).
Table 4
Ocular symptoms described by 6 patients with post-Ebola syndrome, Sierra Leone
| Patient age, y/sex | Symptom |
|---|---|
| 8/F | Eye pain |
| 14/F | Clear eye discharge |
| 20/F | Clear eye discharge |
| 28/F | Red eyes and blurred vision on the left |
| 29/F | Red eyes |
| 46/M | Blurred vision |
Long-term Complications of Ebola Virus Disease (EVD)
Sunday, February 28th, 2016
For Immediate Release: Tuesday, Feb. 23, 2016
Other Findings May Have Implications for Potential Sexual Transmission of Ebola
Preliminary findings from PREVAIL III, a study of Ebola virus disease (EVD) survivors being conducted in Liberia, indicate that both Ebola survivors and their close contacts have a high burden of illness. However, the prevalence of eye, musculoskeletal, and neurological complications was greater among the individuals who survived EVD.
These findings were presented today at the 23rd Conference on Retroviruses and Opportunistic Infections at the John B. Hynes Veterans Memorial Convention Center in Boston.
CDC: Zika virus disease in the United States, 2015–2016 (January 1, 2015 – February 24, 2016)
Saturday, February 27th, 2016Zika virus disease in the United States, 2015–2016
As of February 24, 2016
- As an arboviral disease, Zika virus is nationally notifiable.
- This update from the CDC Arboviral Disease Branch includes provisional data reported to ArboNET for January 1, 2015 – February 24, 2016.
US States
- Travel-associated Zika virus disease cases reported: 107
- Locally acquired vector-borne cases reported: 0
US Territories
- Travel-associated cases reported: 1
- Locally acquired cases reported: 39
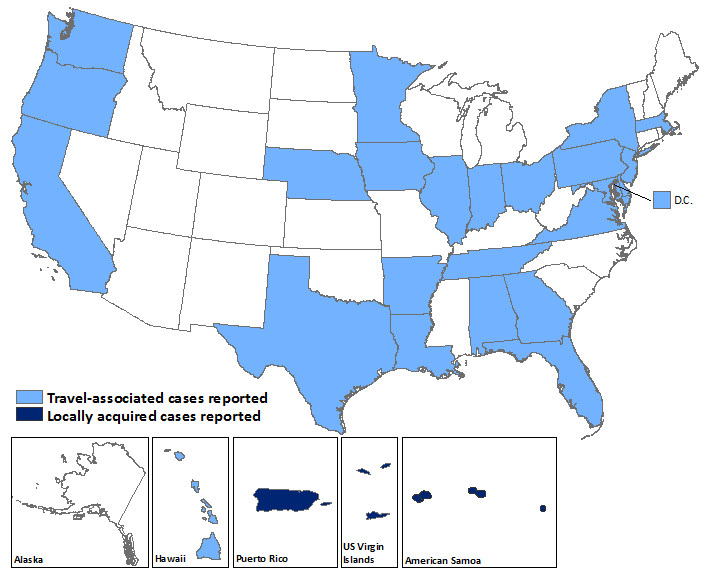
Laboratory-confirmed Zika virus disease cases reported to ArboNET by state or territory — United States, 2015–2016 (as of February 24, 2016)
| States | Travel-associated cases (N=107) | Locally acquired cases (N=0) |
|---|---|---|
| Alabama | 1 | 0 |
| Arkansas | 1 | 0 |
| California | 6 | 0 |
| Delaware | 1 | 0 |
| District of Columbia | 3 | 0 |
| Florida | 28 | 0 |
| Georgia | 1 | 0 |
| Hawaii | 4 | 0 |
| Illinois | 4 | 0 |
| Indiana | 1 | 0 |
| Iowa | 1 | 0 |
| Louisiana | 1 | 0 |
| Maryland | 3 | 0 |
| Massachusetts | 2 | 0 |
| Minnesota | 3 | 0 |
| Nebraska | 2 | 0 |
| New Jersey | 1 | 0 |
| New York | 17 | 0 |
| Ohio | 4 | 0 |
| Oregon | 1 | 0 |
| Pennsylvania | 4 | 0 |
| Tennessee | 1 | 0 |
| Texas | 13 | 0 |
| Virginia | 3 | 0 |
| Washington | 1 | 0 |
| Territories | (N=1) | (N=39) |
| American Samoa | 0 | 4 |
| Puerto Rico | 1 | 34 |
| US Virgin Islands | 0 | 1 |
WHO: Weekly Zika Situation Report, 2/26/2016
Saturday, February 27th, 2016** Between 1 January 2007 and 25 February 2016, a total of 52 countries and territories have reported autochthonous (local) transmission of Zika virus, including those where the outbreak is now over and countries and territories that provided indirect evidence of local transmission.
** Among the 52 countries and territories, Marshall Islands, Saint Vincent and the Grenadines, and Trinidad and Tobago are the latest to report autochthonous transmission of Zika virus. The geographical distribution of Zika virus has steadily widened since the virus was first detected in the Americas in 2015. Autochthonous Zika virus transmission has been reported in 31 countries and territories of this region. Zika virus is likely to be transmitted and detected in other countries within the geographical range of competent mosquito vectors, especially Aedes aegypti.
** So far an increase in microcephaly cases and other neonatal malformations have only been reported in Brazil and French Polynesia, although two cases linked to a stay in Brazil were detected in two other countries.
** During 2015 and 2016, eight countries and territories have reported an increased incidence of Guillain-Barré syndrome (GBS) and/or laboratory confirmation of a Zika virus infection among GBS cases.
** Evidence that neurological disorders, including microcephaly and GBS, are linked to Zika virus infection remains circumstantial, but a growing body of clinical and epidemiological data points towards a causal role for Zika virus.
CDC’s Zika MAC test has been cleared by FDA (Emergency Use Authorization) for detecting evidence of the virus in human sera or CSF
Saturday, February 27th, 2016** the Zika MAC test is used to gauge recent infection and can detect the virus as early as 4 days after symptoms begin.
“….. the Food and Drug Administration (FDA) issue an Emergency Use Authorization (EUA) for emergency use of the Centers for Disease Control and Prevention’s (CDC) Zika Immunoglobulin M (IgM) Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA) for the presumptive detection of Zika virusspecific IgM in human sera or cerebrospinal fluid (CSF)……”
2 confirmed and 4 probable cases of sexual transmission of Zika virus from male travelers to female nontravelers; The cases described here suggest that sexual transmission of Zika virus is more common than previously reported..
Saturday, February 27th, 2016Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission — Continental United States, 2016
Early Release / February 26, 2016 / 65
Summary
What is already known about this topic?Zika virus is spread primarily by Aedes species mosquitoes, though recent reports have described two instances of sexual transmission of Zika virus, and replicative virus has been isolated from semen of one man with hematospermia. CDC released interim guidance for prevention of sexual transmission of Zika virus on February 5, 2016.
What is added by this report?This report provides information on six confirmed and probable cases of sexual transmission of Zika virus from male travelers to female nontravelers. This suggests that sexual transmission of Zika virus might be more common than previously reported.
What are the implications for public health practice?Men who reside in or have traveled to an area of ongoing Zika virus transmission who have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex (i.e., vaginal intercourse, anal intercourse, or fellatio) with their pregnant partner for the duration of the pregnancy.
Zika virus is a flavivirus closely related to dengue, West Nile, and yellow fever viruses. Although spread is primarily by Aedes species mosquitoes, two instances of sexual transmission of Zika virus have been reported (1,2), and replicative virus has been isolated from semen of one man with hematospermia (3). On February 5, 2016, CDC published recommendations for preventing sexual transmission of Zika virus (4). Updated prevention guidelines were published on February 23.* During February 6–22, 2016, CDC received reports of 14 instances of suspected sexual transmission of Zika virus. Among these, two laboratory-confirmed cases and four probable cases of Zika virus disease have been identified among women whose only known risk factor was sexual contact with a symptomatic male partner with recent travel to an area with ongoing Zika virus transmission. Two instances have been excluded based on additional information, and six others are still under investigation. State, territorial, and local public health departments, clinicians, and the public should be aware of current recommendations for preventing sexual transmission of Zika virus, particularly to pregnant women (4). Men who reside in or have traveled to an area of ongoing Zika virus transmission and have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex with their pregnant partner for the duration of the pregnancy (4).
Zika virus disease is an arboviral disease and a nationally notifiable condition in the United States (5). For the purposes of this report, a confirmed or probable case of sexually transmitted Zika virus disease was defined as an illness meeting the confirmed or probable arbovirus surveillance case definition in a person whose only known risk factor was sexual contact with a partner who recently traveled to an area with ongoing Zika virus transmission (6).
During February 6–22, 2016, two confirmed and four probable cases of Zika virus sexual transmission were reported to CDC by health officials from multiple states. Median patient age was 22.5 years (range = 19–55 years), and several women were pregnant. In all cases where type of sexual contact was documented, the contact included condomless vaginal intercourse and occurred when the male partner was symptomatic or shortly after symptoms resolved. Three illustrative cases are presented.
Case 1. In mid-January, immediately after returning to the United States from a 10-day trip to the Caribbean, a man developed illness with fever, arthralgia, bilateral conjunctivitis, and a maculopapular, pruritic rash. The illness lasted 6 days. No hematospermia or prostatitis was noted. On the 1st or 2nd second day of illness, he had condomless vaginal intercourse with his female partner. The woman developed a febrile illness 13–14 days after sexual contact, with rash, conjunctivitis, and myalgia. Zika virus RNA was detected in the woman’s serum by reverse transcription-polymerase chain reaction (RT-PCR) assay. Test results for the man are pending. The woman had no recent history of travel outside of the continental United States, and local mosquito-borne transmission of Zika virus was not considered possible; the vectors that transmit the virus are not present or active where she lives, based on the location and current temperatures.
Case 2. In late January, a man returned to the United States after a 4-week trip to Central America. The same day, he developed fever, arthralgia, generalized pruritus, myalgia, and eye discomfort. He had condomless vaginal intercourse with his female partner several times during the following 8 days. Ten days after the man’s return, his female partner developed fever, pruritic rash, arthralgia, eye pain, photophobia, headache, vomiting, and myalgia. Zika virus infection in the woman was confirmed by RT-PCR testing of serum. Serum collected from the man tested positive for Zika virus immunoglobulin M (IgM) antibodies; confirmation is pending. The woman had no recent history of travel outside the continental United States, and current local mosquito-borne transmission of Zika virus was not considered possible where she lives.
Case 3. In mid-January, a man returned from Central America with fever, rash, arthralgia, conjunctivitis, headache, and myalgia. His symptoms began 3 days earlier and persisted until approximately 3 days after his return. On the day of his return, he had sexual contact with his female partner. Ten days later, the woman developed rash, arthralgia, conjunctivitis, and myalgia. Serum collected from the woman tested positive for Zika virus IgM; confirmation is pending. Test results for the man are pending. The woman had no recent history of travel outside of the continental United States, and current local mosquito-borne transmission of Zika virus was not considered possible where she lives.
Discussion
The cases described here suggest that sexual transmission of Zika virus is more common than previously reported. To date, all reported cases of sexual transmission of Zika virus have been from symptomatic male partners. Sexual transmission of Zika virus from infected women to their sex partners and from persons who are asymptomatically infected has not been reported. Prevention of infection during pregnancy is particularly important because of the growing evidence linking maternal Zika virus infection with congenital microcephaly, fetal loss, and other adverse reproductive health outcomes (7). Whether sexual transmission of Zika virus poses a different risk for congenital infection than that of mosquito-borne transmission is unknown.
Zika virus testing is currently recommended to establish a diagnosis in exposed persons with signs or symptoms consistent with Zika virus disease, and can be offered to asymptomatic pregnant women who have been exposed to Zika virus (8). In these recommendations, exposure has been defined as living in or having traveled to an area with ongoing Zika virus transmission (8). Health care providers should now consider any person who has had condomless sex (i.e., vaginal intercourse, anal intercourse, or fellatio) with a male partner who has traveled to an area of ongoing Zika virus transmission and who has had symptoms of Zika virus disease during travel or within 2 weeks of return as potentially exposed. Routine testing of men who have traveled for the purpose of assessing risk for sexual transmission is not recommended (4).
Men who reside in or have traveled to an area of ongoing Zika virus transmission who have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex with their pregnant partner for the duration of the pregnancy (4). Pregnant women should discuss their male partner’s recent travel history and any illness consistent with Zika virus disease (http://www.cdc.gov/zika/symptoms) with their health care provider; providers can consult CDC’s guidelines for evaluation and testing of pregnant women (4). At this time, the length of time that virus might persist in semen is unknown. A recent report described detection of Zika virus RNA in semen by RT-PCR as long as 62 days after illness onset; however, infectious virus was not cultured from semen (9). Recommendations for prevention of sexual transmission of Zika virus will be updated as new information regarding the risks for transmission becomes available.
References
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011;17:880–2. CrossRef PubMed
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. February 2, 2016. Dallas, TX: Dallas County Health and Human Services; 2016. http://www.dallascounty.org/department/hhs/documents/February2016Newsletter.pdf.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015;21:359–61. CrossRef PubMed
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:120–1. CrossRef PubMed
- Council of State and Territorial Epidemiologists. 2015 National Surveillance Case Definition for Arboviral diseases, neuroinvasive and non-neuroinvasive. Atlanta, GA: Council of State and Territorial Epidemiologists; 2015. http://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/
- Pan American Health Organization. Countries and territories with autochthonous Zika virus transmission in the Americas. Washington, DC: Pan American Health Organization; 2016. http://www.paho.org/hq/index.php?option=com_content&view=article&id=11603&Itemid=41696&lang=en
- Meaney-Delman D, Hills SL, Williams C, et al. Zika virus infection among US pregnant women travelers—August 2015–February 2016. MMWR Morb Mortal Wkly Rep 2016;65.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:122–7. CrossRef PubMed
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen[letter]. Emerg Infect Dis 2016;22. Epub, February 11, 2016. CrossRef
Suggested citation for this article: Hills SL, Russell K, Hennessey M, et al. Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission — Continental United States, 2016. MMWR Morb Mortal Wkly Rep. ePub: 26 February 2016. DOI: http://dx.doi.org/10.15585/mmwr.mm6508e2er.
9 U.S. pregnant travelers with Zika virus infection: Pregnancy outcomes included two early pregnancy losses, two elective terminations, and three live births (2 apparently healthy infants and 1 infant with severe microcephaly); 2 pregnancies (18 weeks’ and 34 weeks’ gestation) are continuing without known complications.
Saturday, February 27th, 2016Zika Virus Infection Among U.S. Pregnant Travelers — August 2015–February 2016
Early Release / February 26, 2016 / 65
Summary
What is already known about this topic?Because of the risk for Zika virus infection and its possible association with adverse pregnancy outcomes, CDC issued a travel alert on January 15, 2016, advising pregnant women to consider postponing travel to areas with ongoing local transmission of Zika virus. CDC also released guidelines for Zika virus testing for pregnant women with a history of travel while pregnant to areas with ongoing Zika virus transmission.
What is added by this report?This report provides preliminary information on testing for Zika virus infection of U.S. pregnant women who had traveled to areas with Zika virus transmission. As of February 17, 2016, nine U.S. pregnant travelers with Zika virus infection had been identified. No Zika virus–related hospitalizations or deaths were reported among pregnant women. Pregnancy outcomes included two early pregnancy losses, two elective terminations, and three live births (two apparently healthy infants and one infant with severe microcephaly); two pregnancies (18 weeks’ and 34 weeks’ gestation) are continuing without known complications.
What are the implications for public health practice?In this small case series, Zika virus infection during pregnancy was associated with a range of outcomes, including early pregnancy losses, congenital microcephaly, and apparently healthy infants. Additional information will be available in the future from a newly established CDC registry for U.S. pregnant women with confirmed Zika virus infection and their infants.
After reports of microcephaly and other adverse pregnancy outcomes in infants of mothers infected with Zika virus during pregnancy, CDC issued a travel alert on January 15, 2016, advising pregnant women to consider postponing travel to areas with active transmission of Zika virus. On January 19, CDC released interim guidelines for U.S. health care providers caring for pregnant women with travel to an affected area (1), and an update was released on February 5 (2). As of February 17, CDC had received reports of nine pregnant travelers with laboratory-confirmed Zika virus disease; 10 additional reports of Zika virus disease among pregnant women are currently under investigation. No Zika virus–related hospitalizations or deaths among pregnant women were reported. Pregnancy outcomes among the nine confirmed cases included two early pregnancy losses, two elective terminations, and three live births (two apparently healthy infants and one infant with severe microcephaly); two pregnancies (approximately 18 weeks’ and 34 weeks’ gestation) are continuing without known complications. Confirmed cases of Zika virus infection were reported among women who had traveled to one or more of the following nine areas with ongoing local transmission of Zika virus: American Samoa, Brazil, El Salvador, Guatemala, Haiti, Honduras, Mexico, Puerto Rico, and Samoa. This report summarizes findings from the nine women with confirmed Zika virus infection during pregnancy, including case reports for four women with various clinical outcomes. U.S. health care providers caring for pregnant women with possible Zika virus exposure during pregnancy should follow CDC guidelines for patient evaluation and management (1,2). Zika virus disease is a nationally notifiable condition. CDC has developed a voluntary registry to collect information about U.S. pregnant women with confirmed Zika virus infection and their infants. Information about the registry is in preparation and will be available on the CDC website.
Zika virus is a mosquito-borne flavivirus that was first isolated from a rhesus monkey in Uganda in 1947 (3). For several decades, only sporadic human disease cases were reported from Africa and Southeast Asia. In 2007, an outbreak was reported on Yap Island, Federated States of Micronesia (3), and outbreaks subsequently were reported from several Pacific Island countries (4). Local transmission of Zika virus was first identified in the Region of the Americas (Americas) in Brazil in May 2015 (5). Since that time, transmission of Zika virus has occurred throughout much of the Americas; as of February 18, a total of 32 countries and territories worldwide have active transmission of Zika virus (http://www.cdc.gov/zika/geo/active-countries.html). Interim guidelines for evaluation and management of pregnant women who have traveled to areas with ongoing local transmission of Zika virus include offering laboratory testing after return from travel (2).
During August 1, 2015–February 10, 2016, CDC received 257 requests for Zika virus testing for pregnant women. Among these requests, 151 (59%) included information indicating that the woman had a clinical illness consistent with Zika virus disease (i.e., two or more of the following signs or symptoms: acute onset of fever, rash, conjunctivitis, or arthralgia). The remaining requests did not document an illness compatible with Zika virus disease, but reporting of symptom information might have been incomplete.
Laboratory confirmation of recent Zika virus infection includes detection of 1) Zika virus, viral RNA, or viral antigen, or 2) Zika virus immunoglobulin M (IgM) antibodies with Zika virus neutralizing antibody titers ≥4-fold higher than neutralizing antibody titers against dengue or other flaviviruses endemic to the region where exposure occurred. Among the 257 pregnant women whose specimens were tested at CDC, 249 (97%) tested negative for recent Zika virus infection and eight (3%) had confirmed Zika virus infection. In addition to the eight patients with laboratory testing performed at CDC, one confirmed case was reported to CDC from a state health department with capacity to test for Zika virus infection.
Among nine pregnant women with confirmed Zika virus disease, no hospitalizations or deaths were reported. All nine women reported at least one of the four most commonly observed symptoms (fever, rash, conjunctivitis, or arthralgia), all women reported rash, and all but one woman had at least two symptoms. Among the six pregnant women with Zika virus disease who reported symptoms during the first trimester, outcomes included two early pregnancy losses, two elective pregnancy terminations, and delivery of a live born infant with microcephaly; one pregnancy is continuing. Among two women with Zika virus infection who had symptoms during the second trimester of pregnancy, one apparently healthy infant has been born and one pregnancy is continuing. One pregnant woman reported symptoms of Zika virus infection in the third trimester of pregnancy, and she delivered a healthy infant.
Selected Case Reports
Patient A. In January 2016, a pregnant woman in her 30s reported symptoms of fever, rash, arthralgia, myalgia, and malaise at 6–7 weeks’ gestation. She had traveled to a Zika-affected area at approximately 5 weeks’ gestation. Serologic testing confirmed recent Zika virus infection. She experienced a spontaneous early pregnancy loss and underwent a dilation and curettage at approximately 8 weeks’ gestation. Products of conception were sent to CDC for testing, and Zika virus RNA was detected by reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemical (IHC) staining (6).
Patient B. In January 2016, a pregnant woman in her 30s underwent laboratory testing for Zika virus infection. She reported a history of travel to a Zika-affected area at approximately 11–12 weeks’ gestation. One day after returning from travel, she developed fever, eye pain, and myalgia. The next day, she developed a rash. Serologic testing confirmed recent Zika virus infection. At approximately 20 weeks’ gestation, she underwent a fetal ultrasound that suggested absence of the corpus callosum, ventriculomegaly, and brain atrophy; subsequent fetal magnetic resonance imaging demonstrated severe brain atrophy. Amniocentesis was performed, and Zika virus RNA was detected by RT-PCR testing. After discussion with her health care providers, the patient elected to terminate her pregnancy.
Patient C. In late 2015, a woman in her 30s gave birth to an infant at 39 weeks’ gestation. The infant’s head circumference at birth was 27 cm (<3rd percentile), indicating severe microcephaly (http://www.cdc.gov/growthcharts/who_charts.htm). After delivery, an epidemiologic investigation revealed that the woman had resided in Brazil until 12 weeks’ gestation. She reported that she had experienced fever, rash, arthralgia, and headache at 7–8 weeks’ gestation. Evidence of Zika virus infection in the mother was confirmed by serologic testing. Molecular and pathologic evaluation of the placenta demonstrated Zika virus RNA by RT-PCR and IHC, respectively. The infant exhibited hypertonia, difficulty swallowing, and seizures, and computerized tomography scan demonstrated multiple scattered and periventricular brain calcifications. Funduscopic examination revealed a pale optic nerve and mild macular chorioretinitis. Newborn hearing screening was normal. The infant was discharged from the hospital with a gastrostomy feeding tube.
Patient D. A pregnant woman in her 30s traveled to a Zika-affected area at approximately 15 weeks’ gestation. She reported symptoms of fever, rash, arthralgia, and headache beginning at the end of her travel (at approximately 17–18 weeks’ gestation). Serologic testing confirmed evidence of Zika virus infection. At approximately 40 weeks’ gestation, she delivered a full-term, apparently healthy infant with no reported abnormalities and a head circumference of 34.5 cm. Cranial ultrasound, newborn hearing screen, and ophthalmologic examination of the infant were all normal.
Discussion
On January 19, 2016, CDC released interim guidelines recommending that pregnant women who had traveled to areas with ongoing local transmission of Zika virus and who had symptoms consistent with Zika virus disease be tested for Zika virus infection (1). These guidelines were updated and expanded on February 5 to offer Zika virus testing to all pregnant women with Zika virus exposure, regardless of the presence of symptoms (2). Although Zika virus testing can be performed in some state, territorial, and local health departments, most testing before mid-February 2016 was performed at CDC. Based on tests performed at CDC as of February 17, 2016, only a small number of pregnant women who reported clinical illness consistent with Zika virus disease had laboratory evidence of a recent Zika virus infection. The combination of clinical signs and symptoms consistent with suspected Zika virus disease, including fever, rash, conjunctivitis, and arthralgia, is not specific to Zika virus disease; there are other causes of this clinical presentation (7). Among the nine pregnant women with Zika virus infection, all reported a clinical illness, including eight women with ≥2 signs and/or symptoms, and one with a generalized rash. The finding of reported clinical illness among all women who tested positive for Zika virus might be related to the initial testing criteria for pregnant women recommended by CDC, which required the presence of clinical illness consistent with Zika virus disease. Additional testing performed as of February 24, 2016 identified no confirmed cases among 162 pregnant women without reported symptoms.
Two women with confirmed Zika virus infection experienced spontaneous pregnancy losses in the first trimester of pregnancy. Although Zika virus RNA was detected in the specimens from both of these cases, it is not known whether Zika virus infection caused the pregnancy losses. First trimester pregnancy loss is common, occurring in approximately 9%–20% of all clinically recognized pregnancies (8), with higher rates in older women. Pregnancy loss has been observed in association with Zika virus infection (6) and after infections with other flaviviruses (e.g., dengue, West Nile, Japanese encephalitis) (9–11); however, a causal relationship has not been established. Additional histopathologic evaluation and RT-PCR testing of tissues from pregnancy losses might provide additional insight into maternal-fetal transmission of Zika virus and the link between maternal-fetal transmission and pregnancy losses.
Seven pregnant women with confirmed Zika virus infection reported fever during pregnancy. Fever has been determined to increase the risk for adverse pregnancy outcomes, including neural tube defects (12). It is not known whether fever might have affected pregnancy outcomes among these pregnant women with Zika virus infection. Because of the potential risks for poor outcomes associated with fever during pregnancy, acetaminophen should be used to treat fever during pregnancy (12).
Approximately half a million pregnant women are estimated to travel to the United States annually from the 32 (as of February 18, 2016) Zika-affected countries and U.S. territories with active transmission of Zika virus (personal communication, Bradley Nelson, February 23, 2016). These numbers might decrease if pregnant women follow CDC recommendations (1) and postpone travel to areas with ongoing local Zika virus transmission. Pregnant women and their partners should also be aware of the risk for Zika virus infection through unprotected sex with an infected male partner, and carefully follow CDC interim guidelines for preventing sexual transmission of Zika virus infection (13). Health care providers should notify their state, local, or territorial health department about women with possible exposure to Zika virus during pregnancy for assistance in arranging testing and interpreting results. CDC has developed a registry to collect information on U.S. pregnant women with confirmed Zika virus infection and their infants. Information gathered from public health officials or health care providers will include clinical information about the pregnancy and the infant at birth and through the first year of life. This voluntary registry has been determined to be a nonresearch public health surveillance activity, and as such, it is not subject to institutional review board requirements. Health care providers are encouraged to discuss participation in the U.S. registry* with pregnant women with Zika virus infection.
References
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:30–3. CrossRef PubMed
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:122–7. CrossRef PubMed
- Duffy MR, Chen T-H, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360:2536–43. CrossRef PubMed
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 2014;20:O595–6. CrossRef PubMed
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—Region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep 2016;65:55–8. CrossRef PubMed
- Martines RB, Bhatnagar J, Keating MK, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65:159–60. CrossRef PubMed
- Roth A, Mercier A, Lepers C, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Euro Surveill 2014;19:20929. CrossRef PubMed
- Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med 1988;319:189–94. CrossRef PubMed
- Chaturvedi UC, Mathur A, Chandra A, Das SK, Tandon HO, Singh UK. Transplacental infection with Japanese encephalitis virus. J Infect Dis 1980;141:712–5. CrossRef PubMed
- O’Leary DR, Kuhn S, Kniss KL, et al. Birth outcomes following West Nile virus infection of pregnant women in the United States: 2003–2004. Pediatrics 2006;117:e537–45. CrossRef PubMed
- Tsai TF. Congenital arboviral infections: something new, something old. Pediatrics 2006;117:936–9. CrossRef PubMed
- Rasmussen SA, Jamieson DJ, Macfarlane K, Cragan JD, Williams J, Henderson Z; Pandemic Influenza and Pregnancy Working Group. Pandemic influenza and pregnant women: summary of a meeting of experts. Am J Public Health 2009;99(Suppl 2):S248–54. CrossRef PubMed
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:120–1. CrossRef PubMed

