Archive for the ‘Anthrax’ Category
Rapid Nanopore Whole-Genome Sequencing for Anthrax Emergency Preparedness
Wednesday, January 22nd, 2020| McLaughlin HP, Bugrysheva JV, Conley AB, Gulvik CA, Cherney B, Kolton CB, et al. Rapid Nanopore Whole-Genome Sequencing for Anthrax Emergency Preparedness. Emerg Infect Dis. 2020;26(2):358-361. https://dx.doi.org/10.3201/eid2602.191351
|
“……as few as 8.5 hours would be required to find evidence of known AMR genes/markers or engineering, including gene insertions and extrachromosomal plasmids from B. anthracis. Although conventional AST remains essential for characterizing functional antimicrobial resistance in B. anthracis, nanopore sequencing provided same-day, on-site genomic characterization useful for an anthrax emergency response.….”
Italy: Unexpected human cases of cutaneous anthrax
Friday, June 14th, 2019. Unexpected human cases of cutaneous anthrax in Latium region, Italy, August 2017: integrated human–animal investigation of epidemiological, clinical, microbiological and ecological factors. Euro Surveill. 2019;24(24):pii=1800685. https://doi.org/10.2807/1560-7917.ES.2019.24.24.1800685
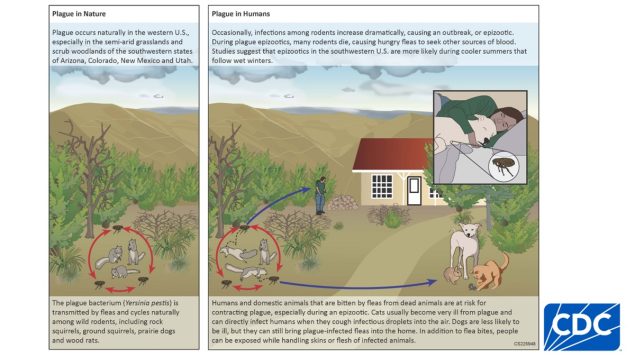
The Scottish island of Gruinard (Anthrax Island): The Island of Death.
Friday, April 5th, 2019“….Over the summer of 1942 and 1943, sheep were placed in open pens and then exposed to bombs, dropped from a Vickers Wellington bomber plane, that scattered anthrax spores across the land. The power of anthrax became quickly clear when the sheep started dying after three days with its potential to cause mass destruction summed up in the report of the tests……”
4/2/1979: The world’s first anthrax epidemic begins in Ekaterinburg, Russia (now Sverdlosk) eventualy killing 62 and seriously injuring another 32
Tuesday, April 2nd, 2019“……workers at the Ekaterinburg weapons plant failed to replace a crucial filter, causing a release of anthrax spores into the outside air. The wind carried the spores to a farming area and infected people and livestock in the area.….”
Tunisia: 31 people to death over the 2014 terrorist attack and a possible anthrax attack in 2019
Friday, March 8th, 2019“……19 letters containing potentially deadly toxins addressed to prominent journalists, politicians and trade unionists, have been intercepted by police at the central Post Office in Tunis and taken for testing.
The National Unit of Investigation for Terrorist Affairs and Organized Crime revealed that the toxic substance was made in Tunisia inside a laboratory.
The Ministry of Interior indicated that it is monitoring the movements of the terrorist cells that plotted the attack, especially that the deadly poison was made with local Tunisian expertise and required huge financial support. …..”
Tunisia: Envelopes delivered to politicians, journalists and syndicate members contained the Anthrax toxin.
Tuesday, March 5th, 2019“……Twenty public figures were targeted in this horrifying terrorist plot, including 10 prominent politicians, seven journalists and activists in syndicates and human rights, they continued.
Moreover, they said that the terrorist groups have shifted their tactics after security measures against them have limited their activity.
For years, they have relied on armed attacks, but they are now forced to resort to poisoning their victims, said the security agencies…..”
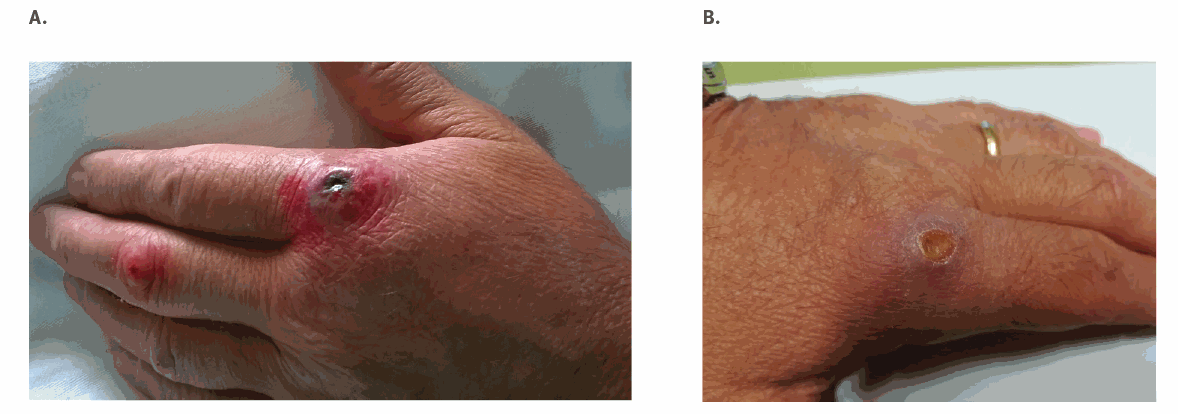
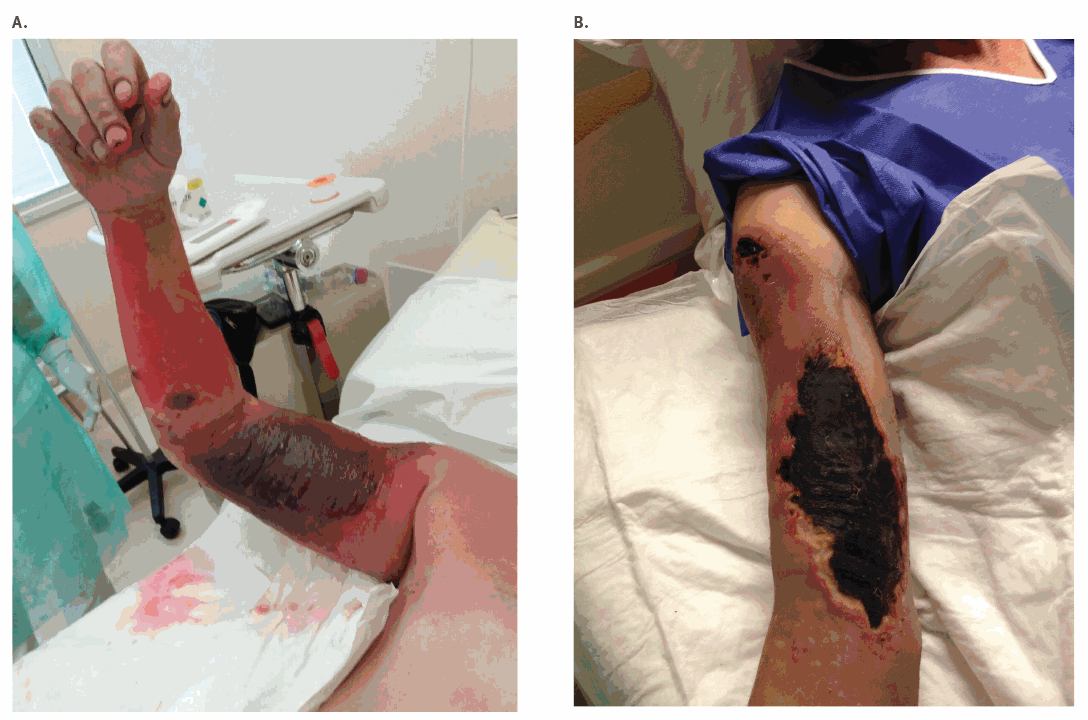
Types of Anthrax(https://www.cdc.gov/anthrax/basics/types/index.html)
An enhanced-delivery anthrax vaccine formulation
Thursday, February 14th, 2019Emergent BioSolutions Inc. announced that Health Canada has approved the company’s New Drug Submission (NDS) for its anthrax vaccine, BioThrax® (Anthrax Vaccine Adsorbed).
Thursday, December 20th, 2018- BioThrax is indicated for active immunization for the prevention of disease caused by Bacillus anthracis, in individuals 18 through 65 years of age, whose occupation or other activities place them at risk of exposure, regardless of the route of exposure.
- BioThrax is administered in a three-dose primary schedule (0, 1 and 6 months) with boosters at three-year intervals recommended thereafter.
Current Emergency Use Authorizations
Thursday, December 6th, 2018
The Emergency Use Authorization (EUA) authority allows FDA to help strengthen the nation’s public health protections against CBRN threats by facilitating the availability and use of MCMs needed during public health emergencies.
Under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), the FDA Commissioner may allow unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when there are no adequate, approved, and available alternatives.
Section 564 of the FD&C Act was amended by the Project Bioshield Act of 2004 and the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), which was enacted in March 2013
Current EUAs
The tables below provide information on current EUAs:
- Anthrax: Doxycycline Mass Dispensing EUA Information and National Postal Model Anthrax EUA Information
- Ebola Virus EUA Information
- Enterovirus D68 (EV-D68) EUA Information
- French Freeze Dried Plasma Information
- H7N9 Influenza EUA Information
- Middle East Respiratory Syndrome Coronavirus (MERS-CoV) EUA Information
- Nerve Agent EUA Information
- Zika Virus EUA Information