Archive for the ‘Leishmaniasis’ Category
Fatal Visceral Leishmaniasis–like Disease
Friday, October 11th, 2019Maruyama et al. Non-Leishmania Parasite in Fatal Visceral Leishmaniasis–like Disease, Brazil. Emerging Infectious Diseases DOI: 10.3201/eid2511.181548 (2019).
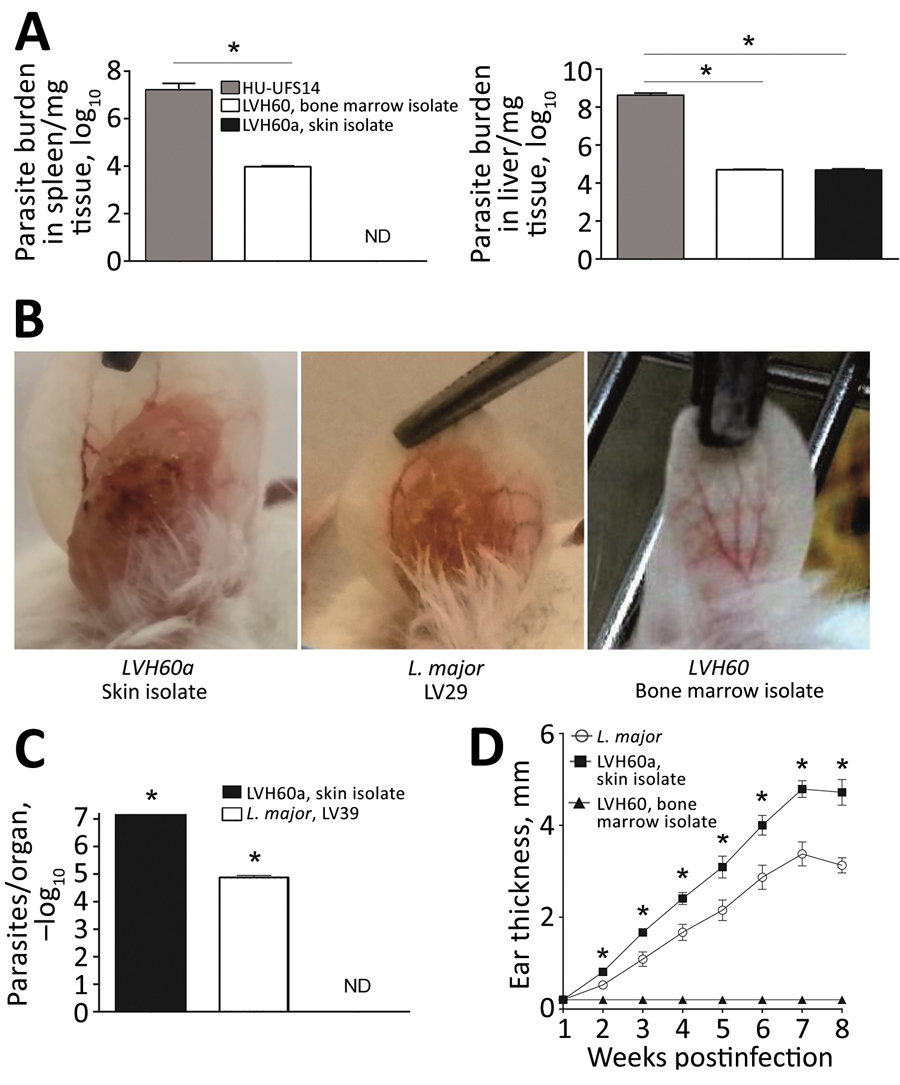
“…..During 2011–2012, we characterized 2 parasite strains, LVH60 and LVH60a, isolated from an HIV-negative man when he was 64 years old and 65 years old (Table; Appendix). Treatment-refractory VL-like disease developed in the man; signs and symptoms consisted of weight loss, fever, anemia, low leukocyte and platelet counts, and severe liver and spleen enlargements. VL was confirmed by light microscopic examination of amastigotes in bone marrow aspirates and promastigotes in culture upon parasite isolation and by a positive rK39 serologic test results. Three courses of liposomal amphotericin B resulted in no response. At the third hospital admission, the illness resembled diffuse cutaneous leishmaniasis, in which several disseminated papular skin lesions were observed (Appendix Figure 1, panel A), and a skin biopsy revealed macrophages filled with amastigotes (Appendix Figure 1, panel B), which his liver biopsy results also showed (Appendix Figure 1, panel C). During this third admission, the LVH60a strain was isolated from the skin. Dermal lesions known as post–kala-azar dermal leishmaniasis (PKDL) have rarely been reported in Brazil (13), and the clinical aspect of the disseminated papular skin lesions on this patient differed from the clinical presentation of PKDL. Because his illness did not respond to therapy, the patient underwent splenectomy. He died of disease and surgical complications...…”
Emerging parasitic disease mimics the symptoms of visceral leishmaniasis in people
Wednesday, October 9th, 2019A new study published this week online in Emerging Infectious Diseases suggests that transmission of a protozoan parasite from insects may also cause leishmaniasis-like symptoms in people. The parasite, however, does not respond to treatment with standard leishmaniasis drugs. The research was conducted by scientists at the Federal Universities of Sergipe and São Carlos, the University of São Paulo, and the Oswaldo Cruz Foundation, all in Brazil, along with investigators at the National Institute of Allergy and Infectious Diseases (NIAID), part of the U.S. National Institutes of Health.
Leishmaniasis is a parasitic disease found in parts of the tropics, subtropics, and southern Europe. It is classified as a neglected tropical disease and is often transmitted by the bite of some sand flies. The most common forms of leishmaniasis are cutaneous, which causes skin sores, and visceral, which affects several internal organs (usually spleen, liver, and bone marrow). According to the World Health Organization, each year between 50,000 and 90,000 people become sick with visceral leishmaniasis (kala-azar), a form of the disease that attacks the internal organs and is fatal in more than 95 percent of cases left untreated. During the last several decades, researchers have described rare cases of patients co-infected with both Leishmania and other groups of protozoan parasites that usually infect insects, including Crithidia. The current study of parasites isolated from a Brazilian patient confirms that Crithidia parasites also can infect people.
 Galway/CDC
Galway/CDC
The 63-year-old patient initially sought treatment for the symptoms of visceral leishmaniasis, including weight loss, fever, anemia, and an enlarged liver and spleen. However, after eight months of standard leishmaniasis treatment, the patient’s symptoms had not improved. The patient developed widespread skin lesions with poorly defined edges (unlike the small lesions with well-defined edges that sometimes appear after treatment for visceral leishmaniasis) and ultimately died.
To determine the cause of disease, researchers cultured parasites taken from the patient’s bone marrow and skin lesions, sequenced their genomes, and discovered that the parasites were not closely related to known disease-causing Leishmania parasites. Instead, they were more closely related to Crithidia fasciculata, a parasite that usually colonizes mosquitoes. To confirm that these Crithidia parasites could infect mammals, the researchers exposed mice to the parasites isolated from the patient, both intravenously and by injection into the skin, and found that both types of parasite infected the liver. The parasites collected from the patient’s skin also caused skin lesions in the mice.
The study raises concerns that the Brazilian patient might not be an isolated case. If Crithidia infections represent an emerging infectious disease in people, there will be an urgent need to develop novel effective treatments, the researchers write. They expressed concern that the disease may be mosquito-borne because Anopheles and Culex mosquitoes can host the Crithidia parasite. More research will be needed to find other human cases, confirm the parasite’s range and host species, and discover potential treatments, the authors note.
Article
Maruyama et al. Non-Leishmania Parasite in Fatal Visceral Leishmaniasis–like Disease, Brazil. Emerging Infectious Diseases DOI: 10.3201/eid2511.181548 (2019).
Illnesses on the rise from mosquito, tick, and flea bites
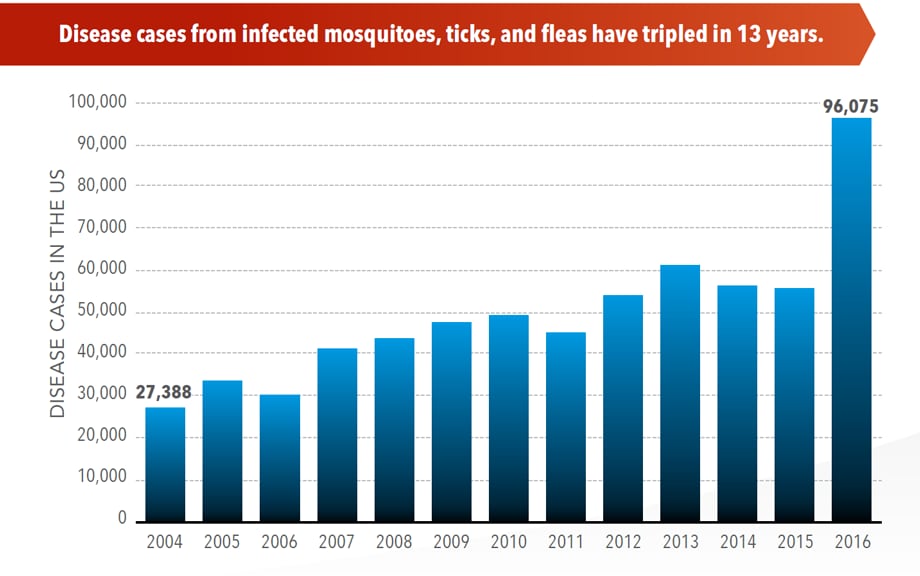
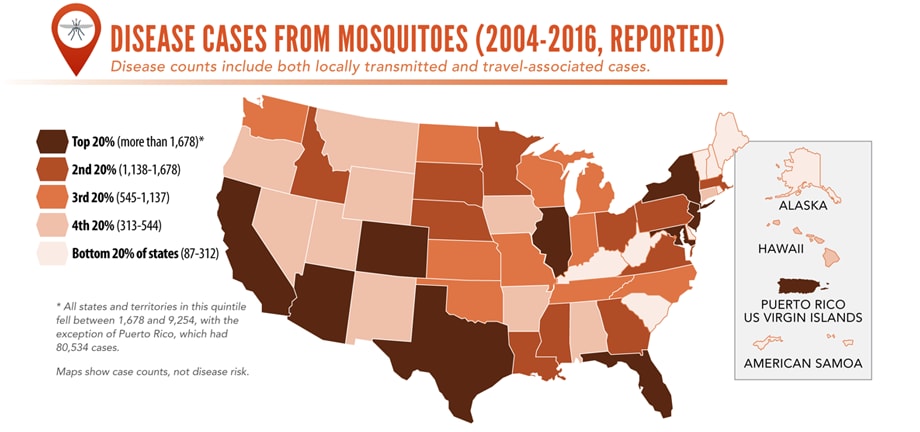
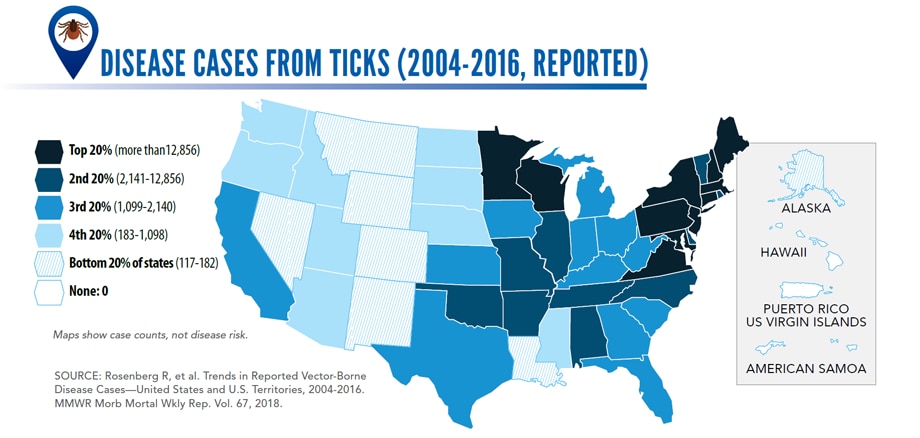
Sunday, June 2nd, 2019Almost everyone has been bitten by a mosquito, tick, or flea. These can be vectors for spreading pathogens (germs). A person who gets bitten by a vector and gets sick has a vector-borne disease, like dengue, Zika, Lyme, or plague. Between 2004 and 2016, more than 640,000 cases of these diseases were reported, and 9 new germs spread by bites from infected mosquitoes and ticks were discovered or introduced in the US. State and local health departments and vector control organizations are the nation’s main defense against this increasing threat. Yet, 84% of local vector control organizations lack at least 1 of 5 core vector control competencies. Better control of mosquitoes and ticks is needed to protect people from these costly and deadly diseases.
State and local public health agencies can
- Build and sustain public health programs that test and track germs and the mosquitoes and ticks that spread them.
- Train vector control staff on 5 core competencies for conducting prevention and control activities. http://bit.ly/2FG1OMwExternal
- Educate the public about how to prevent bites and control germs spread by mosquitoes, ticks, and fleas in their communities.
Increasing threat, limited capacity to respond
More cases in the US (2004-2016)
- The number of reported cases of disease from mosquito, tick, and flea bites has more than tripled.
- More than 640,000 cases of these diseases were reported from 2004 to 2016.
- Disease cases from ticks have doubled.
- Mosquito-borne disease epidemics happen more frequently.
More germs (2004-2016)
- Chikungunya and Zika viruses caused outbreaks in the US for the first time.
- Seven new tickborne germs can infect people in the US.
More people at risk
- Commerce moves mosquitoes, ticks, and fleas around the world.
- Infected travelers can introduce and spread germs across the world.
- Mosquitoes and ticks move germs into new areas of the US, causing more people to be at risk.
The US is not fully prepared
-
- Local and state health departments and vector control organizations face increasing demands to respond to these threats.
- More than 80% of vector control organizations report needing improvement in 1 or more of 5 core competencies, such as testing for pesticide resistance.
- More proven and publicly accepted mosquito and tick control methods are needed to prevent and control these diseases.
New research shows that the geographical range of vector-borne diseases such as chikungunya, dengue fever, leishmaniasis, and tick-borne encephalitis (TBE) is expanding rapidly.
Tuesday, April 16th, 2019“……..Global warming has allowed mosquitoes, ticks and other disease-carrying insects to proliferate, adapt to different seasons, and invade new territories across Europe over the past decade–with accompanying outbreaks of dengue in France and Croatia, malaria in Greece, West Nile Fever in Southeast Europe, and chikungunya virus in Italy and France. …….”
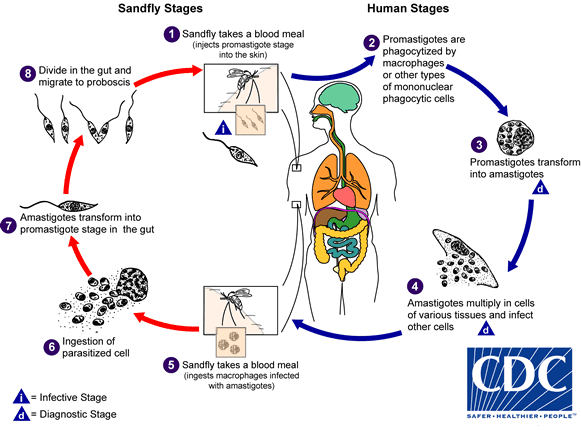
Post-kala-azar dermal leishmaniasis (PKDL): a skin condition that can develop a few months or even years after someone has successfully completed treatment for kala-azar.
Friday, June 8th, 2018“….There are different forms of leishmaniasis, a parasitic disease transmitted by the bite of a sand fly, barely visible to the human eye: visceral leishmaniasis (kala-azar), cutaneous leishmaniasis, and mucocutaneous leishmaniasis. Post-kala-azar dermal leishmaniasis is usually a sequel of visceral leishmaniasis that appears as macular, papular or nodular rash usually on the face, upper arms, and other parts of the body…..”
The Director of the Environmental Health Office in Zliten confirmed that the recorded cases of leishmaniasis this year has increased to 51 persons in the city.
Saturday, April 14th, 2018“….[He] explained that there is no cure at the current time for the disease in Libya, noting that the cost of treatment for this disease is prohibitively expensive and could amount to 10 thousand Libyan dinars per person….. Zliten Municipality warned against the spread of leishmaniasis, declaring that 351 cases were recorded in the city in 2017…..Leishmaniasis is a parasitic disease spread to humans by bites of sandflies that are usually active from May to September each year…..”
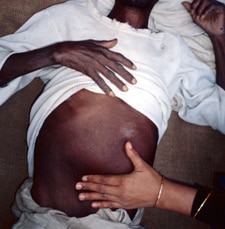 Credit: C. Bern, CDC
Credit: C. Bern, CDC
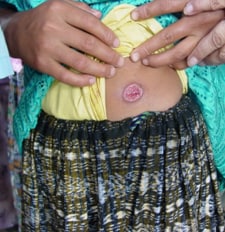 (Credit: B. Arana, MERTU, Guatemala)
(Credit: B. Arana, MERTU, Guatemala)
University of Iowa study: A vaccination used to treat dogs with leishmaniasis could be effective in containing its spread to humans.
Friday, April 13th, 2018“….Leishmaniasis is a tropical infectious disease found in 98 countries around the globe. According to an estimate from the World Health Organization, it kills approximately 20,000 to 40,000 people a year in mostly five countries: Bangladesh, Brazil, Ethiopia, India, and Sudan. It also kills thousands of dogs.….”

CDC’s Emergency Drugs for US Clinicians and Hospitals
Thursday, June 8th, 2017Our Formulary
The following information is provided as an informational resource for guidance only. It is not intended as a substitute for professional judgment. These highlights and any hyperlinks may not include all the information needed to use each respective drug or biologic safely and effectively. See full prescribing information (package insert) or IND protocol for each respective drug or biologic, which accompany the product when it is delivered to the treating physician and/or pharmacist.
The Drug Service formulary is subject to change based on current public health needs, updates to treatment guidelines, and/or drug availability. For historical reference, we have included products no longer supplied by the Drug Service.
| Product & Supplier | Indication & Eligibility | How Supplied |
|---|---|---|
| Anthrax Vaccine Absorbed
(Also known as “AVA”; BioThrax®, Emergent BioSolutions) |
For the active immunization for the prevention of disease caused by Bacillus anthracis, in persons 18 through 65 years of age at high risk for exposure
Because the risk for anthrax infection in the general population is low, routine immunization is not recommended The safety and efficacy of BioThrax® in a post-exposure setting have not been established. |
Suspension for injection in 5 mL multidose vials, each containing 10 doses |
| Artesunate, intravenous
(Supplied to CDC by the Walter Reed Army Institute of Research) |
For the treatment of severe malaria in patients who require parenteral (IV) therapy
Patient must meet the eligibility criteria in the IND protocol |
110 mg; sterile dry-filled powder with phosphate buffer diluent for reconstitution |
| Benznidazole
(Benznidazol, Manufactured by LAFEPE) |
For the treatment of American trypanosomiasis (Chagas disease)
Patient must meet the eligibility criteria in the IND protocol |
100 mg double-scored tablet
12.5 mg dispersible tablet for pediatric use |
| Botulism Antitoxin Heptavalent (Equine), Types A-G
(Also known as “HBAT”; Manufactured by Cangene Corp. – BAT™) |
For the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin | 20 mL or 50 mL single-use glass vial
May be received frozen or thawed |
| Diethylcarbamazine
(Also known as “DEC”; Supplied to CDC by the World Health Organization; Manufactured by E.I.P.I.C.O.) |
For the treatment of certain filarial diseases, including lymphatic filariasis caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori; tropical pulmonary eosinophilia; and loiasis
For prophylactic use in persons determined to be at increased risk for Loa loa infection Patient must meet the eligibility criteria in the IND protocol |
100 mg tablet |
| Diphtheria Antitoxin (Equine)
(Also known as “DAT”; Manufactured by Instituto Butantan) |
For prevention or treatment of actual or suspected cases of diphtheria
Patient must meet the eligibility criteria in the IND protocol |
1 mL single-use ampule containing 10,000 units |
| Eflornithine
(Also known as “DFMO”; Supplied to CDC by the World Health Organization; Manufactured by Sanofi Aventis – Ornidyl®) |
For the treatment of second-stage African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei gambiense, with involvement of the central nervous system | 20 g/100 mL hypertonic solution for IV infusion
Must be diluted with Sterile Water for Injection before use |
| Melarsoprol
(Supplied to CDC by the World Health Organization; Manufactured by Sanofi Aventis – Arsobal®) |
For the treatment of second-stage African trypanosomiasis (sleeping sickness), with involvement of the central nervous system
Patient must meet the eligibility criteria in the IND protocol |
5 mL glass ampule containing 180 mg/5 mL (36 mg/mL) |
| Nifurtimox
(Supplied to CDC by the World Health Organization; Manufactured by Bayer – Lampit®) |
For the treatment of American trypanosomiasis (Chagas disease)
Patient must meet the eligibility criteria in the IND protocol |
120 mg double-scored tablet |
| Sodium Stibogluconate
(Manufactured by GlaxoSmithKline, UK – Pentostam®) |
For the treatment of leishmaniasis
Patient must meet the eligibility criteria in the IND protocol |
Solution for injection in 100 mL multidose bottle
100 mg pentavalent antimony (Sb) per mL |
| Suramin
(Supplied to CDC by the World Health Organization; Manufactured by Bayer – Germanin) |
For the treatment of first-stage African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei rhodesiense, without involvement of the central nervous system
Patient must meet the eligibility criteria in the IND protocol |
1 gram of suramin for injection in a 10 mL vial (100 mg/mL solution of suramin sodium)
Must be reconstituted with 10 mL Sterile Water for Injection before use |
| Vaccinia Vaccine
(Also known as the “Smallpox Vaccine”; Manufactured by Sanofi Aventis – ACAM2000®) |
For active immunization against smallpox disease for persons determined to be at high risk for smallpox infection | Lyophilized powder reconstituted with diluent (provided)
Contains 100 doses per vial |
Anthrax Vaccine Adsorbed
Anthrax Vaccine Adsorbed (AVA) is indicated for the active immunization for the prevention of disease caused by Bacillus anthracis, in persons 18 through 65 years of age at high risk for exposure. The safety and efficacy of AVA in a post-exposure setting have not been established.
CDC provides anthrax vaccine for laboratory workers conducting research under federally funded projects who require preexposure vaccination based on their occupational risk.
Preexposure vaccination is recommended for laboratorians at risk for repeated exposure to fully virulent B. anthracis spores, such as those who 1) work with high concentrations of spores with potential for aerosol production; 2) handle environmental samples that might contain powders and are associated with anthrax investigations; 3) routinely work with pure cultures of B. anthracis; 4) frequently work in spore-contaminated areas after a bioterrorism attack; or 5) work in other settings where repeated exposures to B. anthracis aerosols may occur. Read more[PDF – 36 pages](https://www.cdc.gov/mmwr/pdf/rr/rr5906.pdf).
More Information for Clinicians
CDC’s Anthrax Vaccination Website
Educational Toolkit for Clinicians (from Department of Defense Anthrax Immunization Program)
Full Prescribing Information for BioThrax®
How to Request
Anthrax vaccine must be administered by or under the supervision of the physician who registers with CDC.
Contact the CDC Drug Service for more information.
Artesunate, Intravenous
Artesunate is in the class of medications known as artemesinins, which are derivatives from the “qinghaosu” or sweet wormwood plant (Artemisia annua). Artesunate is not currently licensed by FDA but is made available in the United States under a CDC-sponsored IND protocol for treatment of documented cases of severe malaria(https://www.cdc.gov/malaria/about/index.html) that require parenteral therapy. Read more(https://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html).
More Information for Clinicians
Diagnostic assistance for malaria is available through DPDx.
How to Request
Clinicians who wish to obtain artesunate for severe malaria should contact the CDC Malaria Hotline at 770-488-7788 (M-F, 8am-4:30pm, Eastern time) or, after hours, the CDC Emergency Operations Center (EOC) at 770-488-7100, and request to speak with a CDC Malaria Branch clinician. A Malaria Branch clinician will provide clinical consultation by telephone and, if indicated, authorize the emergency release of artesunate from one of the CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
Requests for unapproved uses cannot be granted.
For non-emergency questions related to artesunate IV, contact the CDC Drug Service.
Benznidazole
Benznidazole is a 2-nitroimidazole trypanocidal agent that was introduced in 1971 for the treatment of Trypanosoma cruzi infection—i.e., Chagas disease, also known as American trypanosomiasis(https://www.cdc.gov/parasites/chagas/). Benznidazole is one of two drugs available from CDC for the treatment of Chagas disease (the other is nifurtimox). In the United States, the need to have drugs available for treating Chagas disease has been increasing, largely because of implementation of T. cruzi blood-donor screening in 2007, which has identified chronically infected persons (mainly Latin American immigrants) who might benefit from treatment and has heightened awareness of Chagas disease.
More Information for Clinicians
Diagnostic assistance for American trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of Chagas disease should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email chagas@cdc.gov) M-F 7:30am-4pm EST.
For emergencies (for example, acute Chagas disease with severe manifestations, Chagas disease in a newborn, or Chagas disease in an immunocompromised person) outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Botulism Antitoxin Heptavalent (Equine), Types A-G
Botulism Antitoxin Heptavalent (HBAT) contains equine-derived antibody to the seven known botulinum toxin types (A-G). HBAT is composed of <2% intact immunoglobulin G (IgG) and ≥90% Fab and F(ab’)2 immunoglobulin fragments. These fragments are created by the enzymatic cleavage and removal of Fc immunoglobulin components in a process sometimes referred to as despeciation. HBAT is supplied on an emergency basis for the treatment of persons thought to be suffering from botulism and works by neutralizing unbound toxin molecules. In 2010, HBAT became the only botulism antitoxin available in the United States for naturally occurring non-infant botulism.
It is available only from CDC because of its limited use and its relatively short expiration date. The antitoxin is stored at CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
BabyBIG® (botulism immune globulin) remains available for infant botulism through the California Infant Botulism Treatment and Prevention Program.
More Information for Clinicians
How to Request
Clinicians who suspect a diagnosis of botulism in a patient should immediately call their state health department’s 24-hour telephone number(https://www.cdc.gov/mmwr/international/relres.html) to maintain effective botulism surveillance and to facilitate rapid detection of outbreaks. The state health department will contact CDC to arrange for a clinical consultation by telephone and, if indicated, release of botulism antitoxin. State health departments requesting botulism antitoxin should contact the CDC Emergency Operations Center (EOC) at 770-488-7100. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5232a8.htm).
For non-emergency questions concerning botulism antitoxin, contact the CDC Drug Service.
Diethylcarbamazine (DEC)
DEC is an antihelminthic agent used for treatment of lymphatic filariasis (caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori), tropical pulmonary eosinophilia, and loiasis(https://www.cdc.gov/parasites/loiasis/); DEC also has prophylactic benefit for Loa loa infection. DEC has been used worldwide for more than 50 years. In the past, Wyeth-Ayerst Laboratories made DEC available as a licensed drug; in the late 1990s, because of unavailability of a bulk chemical supplier, Wyeth-Ayerst discontinued distribution of DEC in the United States.
More Information for Clinicians
Diagnostic assistance for filarial diseases is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of filarial diseases should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
After-hours emergencies: 1-770-488-7100
Diphtheria Antitoxin (Equine)
Diphtheria antitoxin (DAT) is used to prevent or treat diphtheria by neutralizing the toxins produced by Corynebacterium diphtheriae. DAT is a sterile, aqueous solution of the refined and concentrated proteins, chiefly globulins, containing antibodies obtained from the serum of horses that have been immunized against diphtheria toxin. DAT is available under an IND protocol sponsored by CDC and is released only for actual or suspected cases of diphtheria(https://www.cdc.gov/diphtheria/about/index.html). The antitoxin is stored at CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
More Information for Clinicians
CDC’s Vaccine-Related Topics: Diphtheria Antitoxin(https://www.cdc.gov/diphtheria/dat.html)
How to Request
Clinicians who suspect a diagnosis of respiratory diphtheria can obtain DAT by contacting the Emergency Operations Center at 770-488-7100. They will be connected with the diphtheria duty officer, who will provide clinical consultation and, if indicated, initiate the release of diphtheria antitoxin.
For non-emergency questions concerning diphtheria antitoxin, contact the CDC Drug Service.
Eflornithine
Eflornithine is an antitrypanosomal agent that inhibits the enzyme ornithine decarboxylase. Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/); the choice of therapy depends on the infecting subspecies of the parasite and on the stage of the infection. Eflornithine is considered the drug of choice for the treatment of second-stage Trypanosoma brucei gambiense (West African) infection, with involvement of the central nervous system. It is not effective against T. b. rhodesiense (East African) infection (see melarsoprol). Although the manufacturer, Aventis, maintains its US licensure, eflornithine is not commercially available in the United States.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Melarsoprol
Melarsoprol is an organoarsenic compound with trypanocidal effects that has been used outside the United States since 1949. Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/); the choice of therapy depends on the infecting subspecies of the parasite and on the stage of the infection. Melarsoprol is used for the treatment of second-stage infection (involving the central nervous system). It is the only available therapy for second-stage Trypanosoma brucei rhodesiense (East African) infection, whereas eflornithine typically is used for second-stage T. b. gambiense (West African) infection.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Nifurtimox
Nifurtimox is a nitrofuran analog that was introduced in 1965 for the treatment of Trypanosoma cruzi infection—i.e., Chagas disease, also known as American trypanosomiasis(https://www.cdc.gov/parasites/chagas/). Nifurtimox is one of two drugs available from CDC for the treatment of Chagas disease (the other is benznidazole). In the United States, the need to have drugs available for treating Chagas disease has been increasing, largely because of implementation of T. cruzi blood-donor screening in 2007, which has identified chronically infected persons (mainly Latin American immigrants) who might benefit from treatment and has heightened awareness of Chagas disease.
More Information for Clinicians
Diagnostic assistance for American trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of Chagas disease should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email chagas@cdc.gov) M-F 7:30am-4pm EST.
For emergencies (for example, acute Chagas disease with severe manifestations, Chagas disease in a newborn, or Chagas disease in an immunocompromised person) outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Sodium Stibogluconate
Sodium stibogluconate (Pentostam®) is a pentavalent antimony compound used for treatment of leishmaniasis(https://www.cdc.gov/parasites/leishmaniasis). The three main clinical syndromes in humans are visceral, cutaneous, and mucosal leishmaniasis. Pentostam is a well-established antileishmanial agent that has been used in many countries of the world for more than half a century.
More Information for Clinicians
Diagnostic assistance for leishmaniasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of leishmaniasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
After-hours emergencies: 1-770-488-7100
Suramin
Suramin is a negatively charged, high-molecular-weight sulfated naphthylamine. It was introduced in the 1920s for the treatment of African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/). Suramin generally is considered the drug of choice for first-stage Trypanosoma brucei rhodesiense (East African) infection, without involvement of the central nervous system. Pentamidine typically is used for first-stage T. b. gambiense (West African) infection.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Vaccinia Vaccine, “Smallpox Vaccine”
Smallpox vaccine is made of live vaccinia virus derived from plaque purification cloning of Dryvax® (calf lymph vaccine, New York City Board of Health Strain) and grown in African Green Monkey kidney (Vero) cells and tested to be free of adventitious agents. It contains approximately 2.5 – 12.5 x 105 plaque-forming units per dose.
Smallpox was declared globally eradicated in 1980. In 1982, Wyeth Laboratories, the only active manufacturer of licensed vaccinia vaccine in the United States, discontinued production; and, in 1983, distribution to the civilian population was discontinued. Since January 1982, smallpox vaccination has not been required for international travelers, and International Certificates of Vaccination no longer include smallpox vaccination. ACAM2000® is a new-generation smallpox vaccine that was licensed in 2010 for use as a medical countermeasure held by the Strategic National Stockpile.
CDC recommends vaccinia vaccine for laboratory workers who directly handle a) cultures or b) animals contaminated or infected with nonhighly attenuated vaccinia virus, recombinant vaccinia viruses derived from nonhighly attenuated vaccinia strains, or other orthopoxviruses that infect humans (e.g., monkeypox, cowpox, vaccinia, and variola). Other health-care workers (e.g., physicians and nurses) whose contact with nonhighly attenuated vaccinia viruses is limited to contaminated materials (e.g., dressings) and who adhere to appropriate infection control measures are at lower risk for inadvertent infection than laboratory workers. However, because a theoretical risk for infection exists, vaccination can be offered to this group. Read more[PDF – 930KB](https://www.cdc.gov/mmwr/pdf/rr/rr5010.pdf).
More Information for Clinicians
Full Prescribing Information for ACAM2000®[PDF – 11 pages]
ACAM2000® Medication Guide[PDF – 6 pages]
CDC’s Vaccine-Related Topics: Smallpox Vaccine
How to Request
Smallpox vaccine must be administered by or under the supervision of the physician who registers with CDC.
Ancillary supplies, such as bifurcated needles (for administration) and 1 mL tuberculin syringes with 25 gauge x 5/8″ needles (for reconstitution), are supplied with the vaccine.
Contact the CDC Drug Service for more information.
Requests for unapproved uses cannot be granted.
Products No Longer Supplied by Drug Service*
Botulinum Toxoid
Pentavalent (ABCDE) botulinum toxoid is a combination of aluminum phosphate-adsorbed toxoid derived from formalin-inactivated types A, B, C, D, and E botulinum toxins, with formaldehyde and thimerosal used as preservatives. Botulinum toxoid was distributed by CDC under an IND protocol for at-risk persons who were actively working or expected to be working with cultures of Clostridium botulinum or the toxins; in 2011, CDC discontinued its program to supply this vaccine. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6042a3.htm).
Botulinum Antitoxin Types AB & E
In March 2010, CDC announced the availability of a new heptavalent botulinum antitoxin (HBAT, Cangene Corporation). HBAT replaced the licensed bivalent botulinum antitoxin AB and an investigational monovalent botulinum antitoxin E (BAT-AB and BAT-E, Sanofi Pasteur), becoming the only botulinum antitoxin available in the United States for naturally occurring non-infant botulism. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5910a4.htm).
Vaccinia Immune Globulin (VIG)
Vaccinia immune globulin (VIG) is released from the CDC Strategic National Stockpile, if indicated, for the treatment of complications associated with vaccinia vaccination. Clinicians wishing to obtain VIG should contact the Emergency Operations Center (EOC) at 770-488-7100. They will be connected with CDC medical staff who can assist them in the diagnosis and management of patients with suspected complications of vaccinia vaccination.
*this list is not all-inclusive
Use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
WHO: New vector control response seen as game-changer
Friday, June 2nd, 2017The call came from the WHO Director-General in May 2016 for a renewed attack on the global spread of vector-borne diseases.
“What we are seeing now looks more and more like a dramatic resurgence of the threat from emerging and re-emerging infectious diseases,” Dr Margaret Chan told Member States at the Sixty-ninth World Health Assembly. “The world is not prepared to cope.”
Dr Chan noted that the spread of Zika virus disease, the resurgence of dengue, and the emerging threat from chikungunya were the result of weak mosquito control policies from the 1970s. It was during that decade that funding and efforts for vector control were greatly reduced.
‘Vector control has not been a priority’
Dr Ana Carolina Silva Santelli has witnessed this first-hand. As former head of the programme for malaria, dengue, Zika and chikungunya with Brazil’s Ministry of Health, she saw vector-control efforts wane over her 13 years there. Equipment such as spraying machines, supplies such as insecticides and personnel such as entomologists were not replaced as needed. “Vector control has not been a priority,” she said.
Today more than 80% of the world’s population is at risk of vector-borne disease, with half at risk of two or more diseases. Mosquitoes can transmit, among other diseases, malaria, lymphatic filariasis, Japanese encephalitis and West Nile; flies can transmit onchocerciasis, leishmaniasis and human African trypanosomiasis (sleeping sickness); and bugs or ticks can transmit Chagas disease, Lyme disease and encephalitis.
Together, the major vector-borne diseases kill more than 700 000 people each year, with populations in poverty-stricken tropical and subtropical areas at highest risk. Other vector-borne diseases, such as tick-borne encephalitis, are of increasing concern in temperate regions.
Rapid unplanned urbanization, massive increases in international travel and trade, altered agricultural practices and other environmental changes are fuelling the spread of vectors worldwide, putting more and more people at risk. Malnourished people and those with weakened immunity are especially susceptible.
A new approach
Over the past year, WHO has spearheaded a new strategic approach to reprioritize vector control. The Global Malaria Programme and the Department of Control of Neglected Tropical Diseases – along with the Special Programme for Research and Training in Tropical Diseases, have led a broad consultation tapping into the experience of ministries of health and technical experts. The process was steered by a group of eminent scientists and public health experts led by Dr Santelli and Professor Thomas Scott from the Department of Entomology and Nematology at the University of California, Davis and resulted in the Global Vector Control Response (GVCR) 2017–2030.
At its Seventieth session, the World Health Assembly unanimously welcomed the proposed response.
The GVCR outlines key areas of activity that will radically change the control of vector-borne diseases:
- Aligning action across sectors, since vector control is more than just spraying insecticides or delivering nets. That might mean ministries of health working with city planners to eradicate breeding sites used by mosquitoes;
- Engaging and mobilizing communities to protect themselves and build resilience against future disease outbreaks;
- Enhancing surveillance to trigger early responses to increases in disease or vector populations, and to identify when and why interventions are not working as expected; and
- Scaling-up vector-control tools and using them in combination to maximize impact on disease while minimizing impact on the environment.
Specifically, the new integrated approach calls for national programmes to be realigned so that public health workers can focus on the complete spectrum of relevant vectors and thereby control all of the diseases they cause.
Recognizing that efforts must be adapted to local needs and sustained, the success of the response will depend on the ability of countries to strengthen their vector-control programmes with financial resources and staff.
A call to pursue novel interventions aggressively
The GVCR also calls for the aggressive pursuit of promising novel interventions such as devising new insecticides; creating spatial repellents and odour-baited traps; improving house screening; pursuing development of a common bacterium that stops viruses from replicating inside mosquitoes; and modifying the genes of male mosquitoes so that their offspring die early.
Economic development also brings solutions. “If people lived in houses that had solid floors and windows with screens or air conditioning, they wouldn’t need a bednet,” said Professor Scott. “So, by improving people’s standard of living, we would significantly reduce these diseases.”

The call for a more coherent and holistic approach to vector control does not diminish the considerable advances made against individual vector-borne diseases.
Malaria is a prime example. Over the past 15 years, its incidence in sub-Saharan Africa has been cut by 45% – primarily due to the massive use of insecticide-treated bed nets and spraying of residual insecticides inside houses.
But that success has had a down side.
“We’ve been so successful, in some ways, with our control that we reduced the number of public health entomologists – the people who can do this stuff well,” said Professor Steve Lindsay, a public health entomologist at Durham University in Britain. “We’re a disappearing breed.”
The GVCR calls for countries to invest in a vector-control workforce trained in public health entomology and empowered in health care responses.
“We now need more nuanced control – not one-size-fits-all, but to tailor control to local conditions,” Professor Lindsay said. This is needed to tackle new and emerging diseases, but also to push towards elimination of others such as malaria, he said.
Dr Lindsay noted that, under the new strategic approach, individual diseases such as Zika, dengue and chikungunya will no longer be considered as separate threats. “What this represents is not three different diseases, but one mosquito – Aedes aegypti,” said Professor Lindsay.
GVCR dovetails with Sustainable Development Goals
The GVCR will also help countries achieve at least 6 of the 17 Sustainable Development Goals. Of direct relevance are goal 3 on good health and well-being, goal 6 on clean water and sanitation, and goal 11 on sustainable cities and communities.
The GVCR goals are ambitious – to reduce mortality from vector-borne diseases by at least 75% and incidence by at least 60% by 2030 – and to prevent epidemics in all countries.
The annual price tag is US$ 330 million globally, or about 5 cents per person – for workforce, coordination and surveillance costs. This is a modest additional investment in relation to insecticide-treated nets, indoor sprays and community-based activities, which usually exceed US$ 1 per person protected per year.
It also represents less than 10% of what is currently spent each year on strategies to control vectors that spread malaria, dengue and Chagas disease alone. Ultimately, the shift in focus to integrated and locally adapted vector control will save money.
‘A call for action’
Dr Santelli expressed optimism that the GVCR will help ministries of health around the world gain support from their governments for a renewed focus on vector control.
“Most of all, this document is a call for action,” said Dr Santelli, who now serves as deputy director for epidemiology in the Brasilia office of the U.S. Centers for Disease Control and Prevention.
It will not be easy, she predicts. The work to integrate vector-control efforts across different diseases will require more equipment, more people and more money as well as a change in mentality. “The risk of inaction is greater,” said Dr Santelli, “given the growing number of emerging disease threats.” The potential impact of the GVCR is immense: to put in place new strategies that will reduce overall burden and, in some places, even eliminate these diseases once and for all.
CORDS on leishmaniasis
Thursday, April 13th, 2017“…New research out today (9 Feb 2016); the Leishmaniasis Gap Analysis Report and Action Plan carried out in Albania, Jordan and Pakistan during 2015, shows despite its worldwide prevalence and disastrous impact on the lives of millions, leishmaniasis is still very much a growing, neglected disease, mainly affecting impoverished communities, living in poor conditions, without adequate access to shelter, healthcare and medication…..”
“….Leishmaniasis, one of the world’s oldest recorded diseases dating back to the 7th century BC, is an entirely treatable parasitic disease spread by female sandflies. Around 310,000,000 people are estimated to be at risk globally, with around 1.6 million new cases each year across 98 countries. The cutaneous form of the disease can lead to distressing and disfiguring skin ulcers and scarring, while visceral leishmaniasis, which affects organs such as the liver and spleen, is invariably fatal if not treated. Over 40,000 people die from the disease every year, making it the second-largest parasitic killer in the world after malaria.
The purpose of the study is to improve the capacity for the diagnosis, treatment and control of leishmaniasis and other vector borne diseases.
Our research found that;
- leishmaniasis is highly correlated with poverty, malnutrition and other diseases which affect immunity, as well as factors such as crowded living conditions and poor sanitation.
- the real health burden of leishmaniasis remains largely unknown with only 600,000 of the estimated 1.6 million cases each year being diagnosed and treated. This is because those who are most affected are from marginalised communities in rural areas or urban slums, who are unable to seek medical attention because of cost and lack of access to treatment
- in the majority of cases leishmaniasis cannot be transmitted directly from an infected person or animal to another person. Rather it requires the presence of female sandfly vectors to spread the disease. It is not therefore a disease that is likely to spread in areas such as Western Europe, which generally lack sandflies and have healthy populations with good sanitation and access to high-quality healthcare
- leishmaniasis has been ignored largely because of its association with poverty and the limited capacity of governments and aid agencies to deal with its complex epidemiology
- it has also been a low priority for multinational pharmaceutical companies to invest in research to develop effective vaccines and therapies……”