Archive for February, 2016
Japan’s Sakurajima volcano
Monday, February 8th, 2016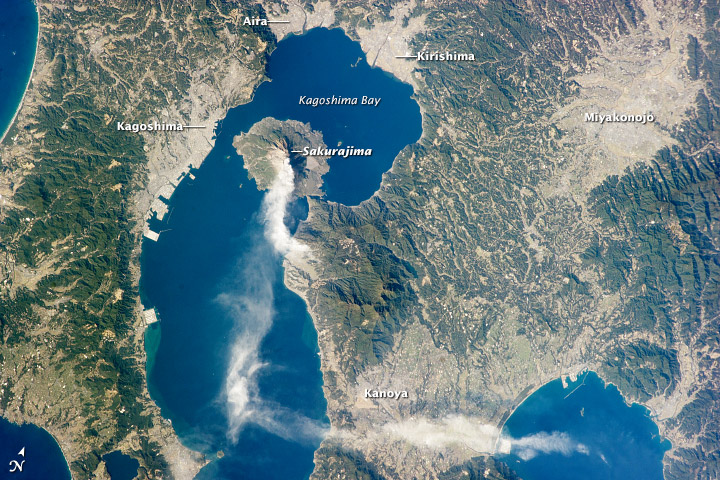
This photograph, taken from the International Space Station, highlights one of Japan’s most active volcanoes. Sakurajima began forming approximately 13,000 years ago; prior to 1914, it was an island in Kagoshima Bay. Sakurajima was joined to the mainland by the deposition of volcanic material following a major eruption in 1914.
Several craters lie near the 1,117-meter summit of Sakurajima. The northernmost crater, Kita-dake, last erupted approximately 5,000 years ago; to the south, Minami-dake and Showa craters have been the site of frequent eruptions since at least the eighth century. The ash plume visible near the volcano’s summit may have originated from either Minami-dake or Showa.
This image highlights the proximity of several large urban areas—Aira, Kagoshima, Kanoya, Kirishima, and Miyakonojo—to Sakurajima. This has prompted studies of potential health hazards presented by the volcanic ash (such as Hillman et al. 2012), and those findings are particularly important if more powerful explosive eruptive activity resumes. The Tokyo Volcanic Ash Advisory Center (VAAC) of the Japan Meteorological Agency issues advisories when eruptions occur. An advisory on the activity in this image was issued less than one hour before the astronaut took the photograph, by which time the plume tail had encountered northeast-trending upper-level winds.
-
Reference
- Hillman, S.E., Horwell, C.J., Densmore, A.L., Damby, D.E., Fubini, B., Ishimine, Y., and Tomatis, M. (2012) Sakurajima volcano: a physico-chemical study of the health consequences of long-term exposure to volcanic ash. Bulletin of Volcanology, 74:913–930.
Turkey’s deputy premier says his country has reached the end of its “capacity to absorb” refugees but will continue to take them in.
Monday, February 8th, 2016** The deputy premier also said that Turkey is hosting a total of 3 million refugees, including 2.5 million Syrians.
CDC Update: Interim Guidelines for Health Care Providers Caring for Pregnant Women and Women of Reproductive Age with Possible Zika Virus Exposure — United States, 2016
Sunday, February 7th, 2016
Update: Interim Guidelines for Health Care Providers Caring for Pregnant Women and Women of Reproductive Age with Possible Zika Virus Exposure — United States, 2016
Early Release / February 5, 2016 / 65(05);1–6
Titilope Oduyebo, MD1,2; Emily E. Petersen, MD2; Sonja A. Rasmussen, MD3; Paul S. Mead, MD4; Dana Meaney-Delman, MD5; Christina M. Renquist, MPH6; Sascha R. Ellington, MSPH2; Marc Fischer, MD4; J. Erin Staples, MD, PhD4; Ann M. Powers, PhD4; Julie Villanueva, PhD4; Romeo R. Galang, MD1,7; Ada Dieke, DrPH1,2; Jorge L. Muñoz, PhD4; Margaret A. Honein, PhD6; Denise J. Jamieson, MD2
CDC has updated its interim guidelines for U.S. health care providers caring for pregnant women during a Zika virus outbreak (1). Updated guidelines include a new recommendation to offer serologic testing to asymptomatic pregnant women (women who do not report clinical illness consistent with Zika virus disease) who have traveled to areas with ongoing Zika virus transmission. Testing can be offered 2–12 weeks after pregnant women return from travel. This update also expands guidance to women who reside in areas with ongoing Zika virus transmission, and includes recommendations for screening, testing, and management of pregnant women and recommendations for counseling women of reproductive age (15–44 years). Pregnant women who reside in areas with ongoing Zika virus transmission have an ongoing risk for infection throughout their pregnancy. For pregnant women with clinical illness consistent with Zika virus disease,* testing is recommended during the first week of illness. For asymptomatic pregnant women residing in areas with ongoing Zika virus transmission, testing is recommended at the initiation of prenatal care with follow-up testing mid-second trimester. Local health officials should determine when to implement testing of asymptomatic pregnant women based on information about levels of Zika virus transmission and laboratory capacity. Health care providers should discuss reproductive life plans, including pregnancy intention and timing, with women of reproductive age in the context of the potential risks associated with Zika virus infection.
Zika virus is primarily transmitted by Aedes aegypti mosquitoes, which are found throughout much of the region of the Americas, including parts of the United States (2,3). These mosquitoes can also transmit dengue and chikungunya viruses (4). The Zika virus outbreak continues to spread (http://www.cdc.gov/zika/geo/index.html), with ongoing Zika virus transmission recently reported in U.S. territories. Evidence suggesting an association of Zika virus infection with an increased risk for congenital microcephaly and other abnormalities of the brain and eye (5) prompted the World Health Organization to declare the Zika virus outbreak a Public Health Emergency of International Concern on February 1, 2016 (http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/).
There is currently no vaccine or medication to prevent Zika virus infection. All travelers to or residents of areas with ongoing Zika virus transmission should be advised to strictly follow steps to avoid mosquito bites because of the potential for exposure to Zika, dengue, and chikungunya viruses (6). Aedes vector mosquitoes bite mostly during daylight hours; thus, protection from mosquito bites is required throughout the day (7). Prevention of mosquito bites includes wearing long-sleeved shirts, pants, permethrin-treated clothing, and using United States Environmental Protection Agency (EPA)-registered insect repellents. Insect repellents containing ingredients such as DEET, picaridin, and IR3535 are safe for use during pregnancy when used in accordance with the product label (6). To prevent human-to-mosquito-to-human transmission, persons infected with Zika, dengue, or chikungunya virus should protect themselves from mosquito exposure during the first week of illness. The number of mosquitoes in and around homes can be reduced by emptying standing water from containers, installing or repairing screens on windows and doors, and using air conditioning if available. Further information on preventing mosquito bites is available online (http://www.cdc.gov/features/stopmosquitoes/).
Antiviral treatment is not currently available for Zika virus disease; treatment is supportive and includes rest, fluids, and analgesic and antipyretic medications. Aspirin and other nonsteroidal anti-inflammatory medications should be avoided until dengue virus infection can be ruled out (8). Dengue virus infection can cause serious complications, including hemorrhage and death, which might be substantially reduced by early recognition and supportive treatment (4,8). Pregnant women with fever should be treated with acetaminophen (9).
Updated Recommendations for Testing Pregnant Women with a History of Travel to Areas with Ongoing Zika Virus Transmission
Recommendations for Zika virus testing of pregnant women who have a clinical illness consistent with Zika virus disease during or within 2 weeks of travel to areas with ongoing Zika virus transmission are unchanged from CDC recommendations released January 19, 2016 (1). Zika virus testing of maternal serum includes reverse transcription-polymerase chain reaction (RT-PCR) testing for symptomatic patients with onset of symptoms during the previous week; immunoglobulin M (IgM) and plaque-reduction neutralizing antibody testing should be performed on specimens collected ≥4 days after onset of symptoms (Figure 1) (1,10).
Serologic testing for Zika virus can be offered to asymptomatic pregnant women who traveled to an area with ongoing Zika virus transmission (Figure 1); however, interpretation of results is complex. Because of cross-reactivity among related flaviviruses, such as dengue, yellow fever, and West Nile viruses, a positive IgM result can be difficult to interpret. Plaque-reduction neutralization testing (PRNT) can be performed to measure virus-specific neutralizing antibodies to Zika virus and other flaviviruses. The levels of neutralizing antibodies can then be compared between flaviviruses, but these tests might also be difficult to interpret in persons who were previously infected with or vaccinated against flaviviruses. However, a negative IgM result obtained 2–12 weeks after travel would suggest that a recent infection did not occur and could obviate the need for serial ultrasounds. Based on experience with other flaviviruses, IgM antibodies will be expected to be present at least 2 weeks after virus exposure and persist for up to 12 weeks (11–14). Information about the performance of serologic testing of asymptomatic persons is limited; a negative serologic test result obtained 2–12 weeks after travel cannot definitively rule out Zika virus infection. Given these challenges in interpreting serologic test results, health care providers should contact their state, local, or territorial health department for assistance with arranging testing and interpreting results. CDC is working with health departments and other organizations to rapidly increase the availability of testing for Zika virus.
Guidelines for Pregnant Women Residing in Areas with Ongoing Zika Virus Transmission
Pregnant women who reside in areas with ongoing Zika virus transmission should be evaluated for symptoms of Zika virus disease. For women who report clinical illness consistent with Zika virus disease, testing by RT-PCR should be performed on serum collected within 7 days of symptom onset. Because viremia decreases over time, a negative RT-PCR result from serum collected 5–7 days after symptom onset does not exclude Zika virus infection, and serologic testing should be performed. (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf).
A false positive IgM result is more likely among women residing in areas with ongoing Zika virus transmission than among travelers because of a higher likelihood of previous exposure to a related flavivirus. Pregnant women who do not report clinical illness consistent with Zika virus disease can be offered IgM testing upon initiation of prenatal care; among women with negative IgM results, repeat testing can be considered in the mid-second trimester because of the ongoing risk for Zika virus exposure and infection throughout pregnancy (Figure 2).
Pregnant women with negative Zika virus IgM testing should receive routine prenatal care, including an assessment of pregnancy dating and an ultrasound at 18–20 weeks of gestation to assess fetal anatomy (15). The ultrasound should include careful evaluation of the fetus for brain anomalies, including microcephaly and intracranial calcifications. Because fetal microcephaly is most easily detected in the late second and early third trimesters of pregnancy (16), and because of ongoing potential exposure to Zika virus, health care providers might consider an additional fetal ultrasound later in pregnancy.
Findings of fetal microcephaly or intracranial calcifications on prenatal ultrasound should prompt health care providers to repeat maternal IgM testing and consider amniocentesis, depending on gestational age. Zika virus testing can be performed on amniotic fluid using RT-PCR to inform clinical management (5). Based on experience with other congenital infections and a small number of prenatally-diagnosed fetal Zika virus infections (5,17), amniocentesis can be used to diagnose intrauterine infections (18). However, the performance of RT-PCR testing of amniotic fluid for Zika virus infection has not been evaluated. Furthermore, the risk for microcephaly or other anomalies when Zika virus RNA is detected in amniotic fluid is not known.
Serial fetal ultrasounds should be considered to monitor fetal anatomy and growth every 3–4 weeks in pregnant women with positive or inconclusive Zika virus test results, and referral to a maternal-fetal medicine specialist is recommended. Testing is recommended at the time of delivery, including histopathologic examination of the placenta and umbilical cord, testing of frozen placental tissue and cord tissue for Zika virus RNA, and testing of cord serum (1,19). Guidelines for infants whose mothers have possible Zika virus infection are available (19). If a pregnant woman with Zika virus disease experiences a fetal loss, Zika virus RT-PCR and immunohistochemical staining should be performed on fetal tissues, including umbilical cord and placenta (1).
Sexual transmission of Zika virus can occur, although there is limited data about the risk (20). The risk for sexual transmission of Zika virus can be eliminated by abstinence and reduced by correct and consistent use of condoms (21). Given the potential risks of maternal Zika virus infection, pregnant women whose male partners have or are at risk for Zika virus infection should consider using condoms or abstaining from sexual intercourse (21). Additional studies are needed to characterize the risk for sexual transmission of Zika virus; recommendations will be updated as more information becomes available.
Special Considerations for Women of Reproductive Age Residing in Areas of Ongoing Zika Virus Transmission
CDC recommends that health care providers discuss pregnancy intention and reproductive options with women of reproductive age. Decisions regarding the timing of pregnancies are personal and complex; reproductive life plans can assist in making these decisions (22). Patient age, fertility, reproductive and medical history, as well as the values and preferences of the woman and her partner should be considered during discussions regarding pregnancy intentions and timing. In the context of the ongoing Zika virus transmission, preconception care should include a discussion of the signs and symptoms and the potential risks associated with Zika virus infection.
Health care providers should discuss strategies to prevent unintended pregnancy with women who do not want to become pregnant; these strategies should include counseling on family planning and use of contraceptive methods. Safety, effectiveness, availability, and acceptability should be considered when selecting a contraceptive method (23). Approximately half of U.S. pregnancies each year are unintended (24); patients should be counseled to use the most effective contraceptive method that can be used correctly and consistently. For women desiring highly effective contraception, long acting reversible contraception, including contraceptive implants and intrauterine devices, might be the best choice (http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/PDF/Contraceptive_methods_508.pdf). When choosing a contraceptive method, the risk for sexually transmitted infections should also be considered; correct and consistent use of condoms reduces the risk for sexually transmitted infections.
Strategies to prevent mosquito bites should be emphasized for women living in areas with ongoing Zika virus transmission who want to become pregnant. These strategies, including wearing pants and long-sleeved shirts, using FDA-approved insect repellents, ensuring that windows and doors have screens, and staying inside air conditioned spaces when possible, can reduce the risk for Zika virus infection and other vector-borne diseases. During preconception counseling visits, the potential risks of Zika virus infection acquired during pregnancy should be discussed.
Women of reproductive age with current or previous laboratory-confirmed Zika virus infection should be counseled that there is no evidence that prior Zika virus infection poses a risk for birth defects in future pregnancies (7). This is because the viremia is expected to last approximately 1 week in patients with clinical illness (2,25). There is no current evidence to suggest that a fetus conceived after maternal viremia has resolved would be at risk for fetal infection (7).
References
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:30–3. CrossRef PubMed
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009;15:1347–50. CrossRef PubMed
- CDC. Chikungunya virus. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html.
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva, Switzerland: World Health Organization; 2009. http://apps.who.int/iris/bitstream/10665/44188/1/9789241547871_eng.pdf.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016;47:6–7. CrossRef PubMed
- CDC. West Nile virus: insect repellent use & safety. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. http://www.cdc.gov/westnile/faq/repellent.html.
- CDC. Zika virus. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. http://www.cdc.gov/zika/index.html.
- CDC. Dengue virus. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. http://www.cdc.gov/Dengue/.
- Rasmussen SA, Kissin DM, Yeung LF, et al. ; Pandemic Influenza and Pregnancy Working Group. Preparing for influenza after 2009 H1N1: special considerations for pregnant women and newborns. Am J Obstet Gynecol 2011;204(Suppl 1):S13–20. CrossRef PubMed
- Division of Vector-Borne Diseases. Arboviral Diseases and Dengue Branches. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf.
- Babaliche P, Doshi D. Catching dengue early: clinical features and laboratory markers of dengue virus infection. J Assoc Physicians India 2015;63:38–41. PubMed
- Wahala WMPB, de Silva AM. The human antibody response to dengue virus infection. Viruses 2011;3:2374–95. CrossRef PubMed
- Gibney KB, Edupuganti S, Panella AJ, et al. Detection of anti-yellow fever virus immunoglobulin m antibodies at 3–4 years following yellow fever vaccination. Am J Trop Med Hyg 2012;87:1112–5. CrossRef PubMed
- Roehrig JT, Nash D, Maldin B, et al. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed west nile virus encephalitis cases. Emerg Infect Dis 2003;9:376–9. CrossRef PubMed
- American Academy of Pediatrics/American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 7th ed. Elk Grove Village, IL: American Academy of Pediatrics/American College of Obstetricians and Gynecologists; 2012.
- Bromley B, Benacerraf BR. Difficulties in the prenatal diagnosis of microcephaly. J Ultrasound Med 1995;14:303–6. PubMed
- American College of Obstetricians and Gynecologists. Practice bulletin no. 151: Cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 2015;125:1510–25. CrossRef PubMed
- Picone O, Costa JM, Leruez-Ville M, Ernault P, Olivi M, Ville Y. Cytomegalovirus (CMV) glycoprotein B genotype and CMV DNA load in the amniotic fluid of infected fetuses. Prenat Diagn 2004;24:1001–6. CrossRef PubMed
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7. CrossRef PubMed
- Foy BD, Kobylinski KC, Foy JLC, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011;17:880–2. CrossRef PubMed
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 2015;65(5).
- CDC. Reproductive life plan tool for health professionals. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. http://www.cdc.gov/preconception/rlptool.html.
- Division of Reproductive Health. National Center for Chronic Disease Prevention. U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013;62(RR-05).
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014;104(Suppl 1):S43–8. CrossRef PubMed
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232–9. CrossRef PubMed
* Clinical illness consistent with Zika virus disease is defined as two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis.
 FIGURE 1. Updated Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman with history of travel to an area with ongoing Zika virus transmission
FIGURE 1. Updated Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman with history of travel to an area with ongoing Zika virus transmission
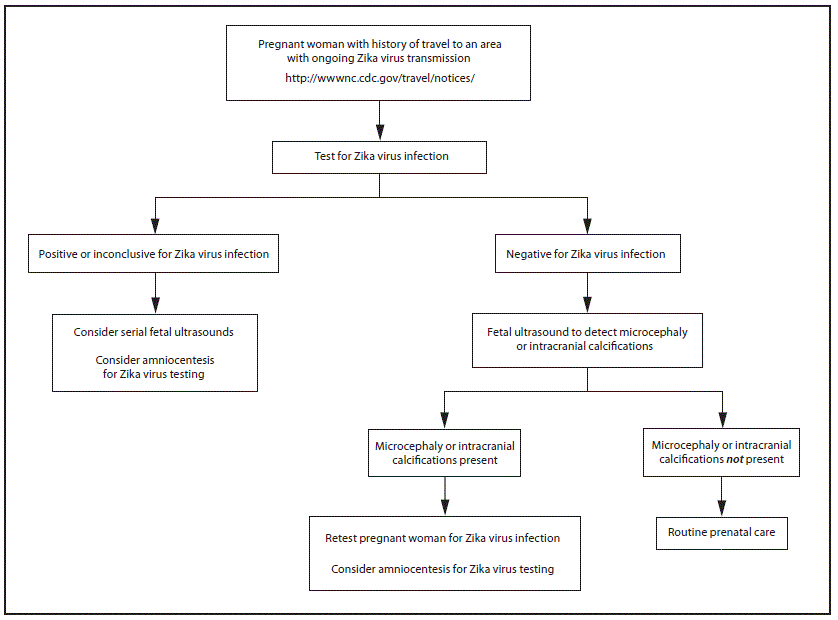 * Testing is recommended for pregnant women with clinical illness consistent with Zika virus disease, which includes two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis during or within 2 weeks of travel. Testing includes Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended.
* Testing is recommended for pregnant women with clinical illness consistent with Zika virus disease, which includes two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis during or within 2 weeks of travel. Testing includes Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended.
† Testing can be offered to pregnant women without clinical illness consistent with Zika virus disease. If performed, testing should include Zika virus IgM, and if IgM test result is positive or indeterminate, neutralizing antibodies on serum specimens. Testing should be performed 2–12 weeks after travel.
§ Laboratory evidence of maternal Zika virus infection: 1) Zika virus RNA detected by RT-PCR in any clinical specimen; or 2) positive Zika virus IgM with confirmatory neutralizing antibody titers that are ≥4-fold higher than dengue virus neutralizing antibody titers in serum. Testing is considered inconclusive if Zika virus neutralizing antibody titers are <4-fold higher than dengue virus neutralizing antibody titers.
¶ Fetal ultrasounds might not detect microcephaly or intracranial calcifications until the late second or early third trimester of pregnancy.
** Amniocentesis is not recommended until after 15 weeks of gestation. Amniotic fluid should be tested for Zika virus RNA by RT-PCR. The sensitivity and specificity of RT-PCR testing on amniotic fluid are not known.
 FIGURE 2. Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman residing in an area with ongoing Zika virus transmission,†† with or without clinical illness consistent with Zika virus disease§§
FIGURE 2. Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman residing in an area with ongoing Zika virus transmission,†† with or without clinical illness consistent with Zika virus disease§§
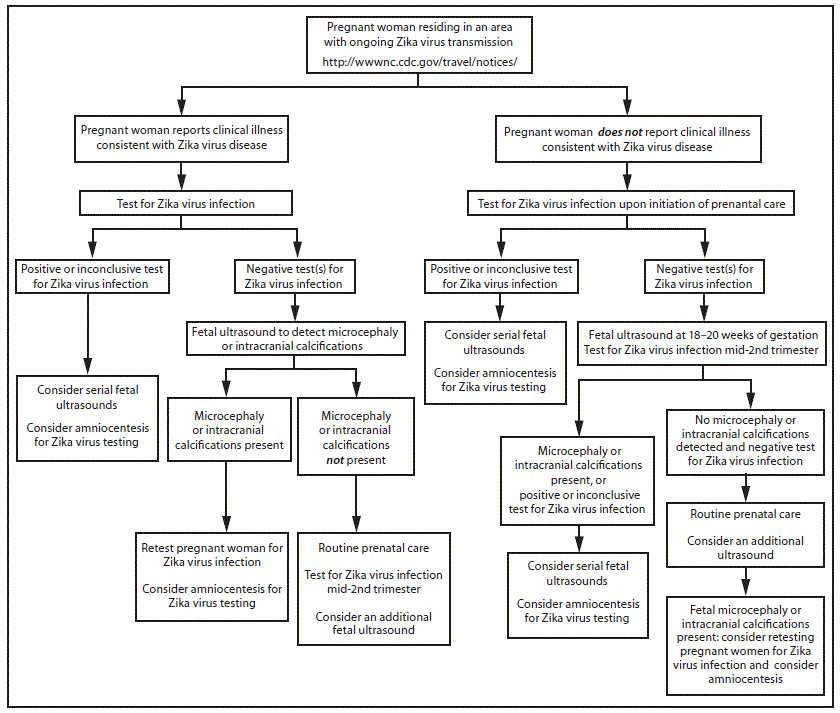 * Tests for pregnant women with clinical illness consistent with Zika virus disease include Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended. If chikungunya or dengue virus RNA is detected, treat in accordance with existing guidelines. Timely recognition and supportive treatment for dengue virus infections can substantially lower the risk of medical complications and death. Repeat Zika virus testing during pregnancy is warranted if clinical illness consistent with Zika virus disease develops later in pregnancy.
* Tests for pregnant women with clinical illness consistent with Zika virus disease include Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended. If chikungunya or dengue virus RNA is detected, treat in accordance with existing guidelines. Timely recognition and supportive treatment for dengue virus infections can substantially lower the risk of medical complications and death. Repeat Zika virus testing during pregnancy is warranted if clinical illness consistent with Zika virus disease develops later in pregnancy.
† Testing can be offered to pregnant women without clinical illness consistent with Zika virus disease. If performed, testing should include Zika virus IgM, and if IgM test result is positive or indeterminate, neutralizing antibodies on serum specimens. Results from serologic testing are challenging to interpret in areas where residents have had previous exposure to other flaviviruses (e.g., dengue, yellow fever).
§ Laboratory evidence of maternal Zika virus infection: 1) Zika virus RNA detected by RT-PCR in any clinical specimen; or 2) positive Zika virus IgM with confirmatory neutralizing antibody titers that are ≥4-fold higher than dengue virus neutralizing antibody titers in serum. Testing is considered inconclusive if Zika virus neutralizing antibody titers are <4-fold higher than dengue virus neutralizing antibody titer.
¶ Amniocentesis is not recommended until after 15 weeks gestation. Amniotic fluid should be tested for Zika virus RNA by RT-PCR. The sensitivity and specificity of RT-PCR testing on amniotic fluid are not known.
** Fetal ultrasounds might not detect microcephaly or intracranial calcifications until the late second or early third trimester of pregnancy.
†† Local health officials should determine when to implement testing of asymptomatic pregnant women based on information about levels of Zika virus transmission and laboratory capacity.
§§ Clinical illness consistent with Zika virus disease is defined as two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis.
Suggested citation for this article: Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: Interim Guidelines for Health Care Providers Caring for Pregnant Women and Women of Reproductive Age with Possible Zika Virus Exposure — United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65(Early Release):1–6. DOI: http://dx.doi.org/10.15585/mmwr.mm6505e2er.
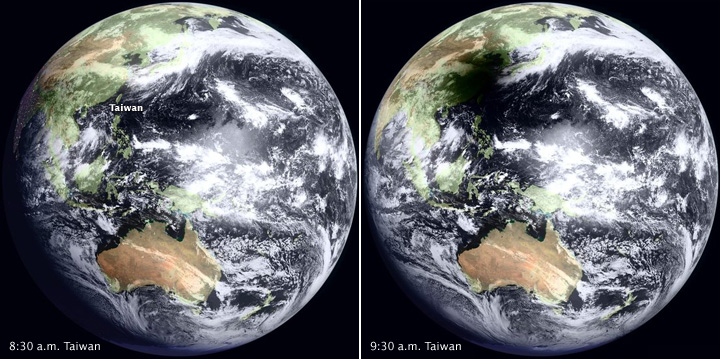
Taiwan: Death toll up to 26 people and more than 100 remain missing in the high-rise apartment building’s rubble
Sunday, February 7th, 2016Pakistan: A man rammed his explosive-laden motorcycle into a Pakistani security forces’ vehicle killing at least 8 and injuring about 20 more.
Sunday, February 7th, 2016The Tainan EQ: Collapse of a high-rise residential complex, killing at least 7 and sending scores to hospitals.
Saturday, February 6th, 2016** Rescuers pulled 249 survivors from the rubble in Tainan
** More than 1,200 firefighters scrambled with ladders, cranes and other equipment to the ruins of a 17-floor residential building \
** It folded like an accordion onto its side.
The 6.4M earthquake struck about 30 miles east of Tainan and a 16-story residential building collapsed
Saturday, February 6th, 2016** UN: More than 200 million girls and women globally have suffered genital mutilation, far higher than previously estimated.
Saturday, February 6th, 2016** “…..half of girls and women who have been cut live in just three countries – Egypt, Ethiopia and Indonesia…..”

** United Nations’ International Day of Zero Tolerance for Female Genital Mutilation
Saturday, February 6th, 2016NEW YORK, 5 February 2016 – At least 200 million girls and women alive today have undergone female genital mutilation in 30 countries, according to a new statistical report published ahead of the United Nations’ International Day of Zero Tolerance for Female Genital Mutilation.
Female Genital Mutilation/Cutting: A Global Concern notes that half of the girls and women who have been cut live in three countries – Egypt, Ethiopia and Indonesia – and refers to smaller studies and anecdotal accounts that provide evidence FGM is a global human rights issue affecting girls and women in every region of the world.
Female genital mutilation refers to a number of procedures. Regardless of which form is practiced, FGM is a violation of children’s rights.
“Female genital mutilation differs across regions and cultures, with some forms involving life-threatening health risks. In every case FGM violates the rights of girls and women. We must all accelerate efforts – governments, health professionals, community leaders, parents and families – to eliminate the practice,” said UNICEF Deputy Executive Director Geeta Rao Gupta.
According to the data, girls 14 and younger represent 44 million of those who have been cut, with the highest prevalence of FGM among this age in Gambia at 56 per cent, Mauritania 54 per cent and Indonesia where around half of girls aged 11 and younger have undergone the practice. Countries with the highest prevalence among girls and women aged 15 to 49 are Somalia 98 per cent, Guinea 97 per cent and Djibouti 93 per cent.
In most of the countries the majority of girls were cut before reaching their fifth birthdays.
The global figure in the FGM statistical report includes nearly 70 million more girls and women than estimated in 2014.This is due to population growth in some countries and nationally representative data collected by the Government of Indonesia. As more data on the extent of FGM become available the estimate of the total number of girls and women who have undergone the practice increases. As of 2016 30 countries have nationally representative data on the practice.
“Determining the magnitude of female genital mutilation is essential to eliminating the practice. When governments collect and publish national statistics on FGM they are better placed to understand the extent of the issue and accelerate efforts to protect the rights of millions of girls and women,” said Rao Gupta.
Momentum to address female genital mutilation is growing. FGM prevalence rates among girls aged 15 to 19 have declined, including by 41 percentage points in Liberia, 31 in Burkina Faso, 30 in Kenya and 27 in Egypt over the last 30 years.
Since 2008, more than 15,000 communities and sub-districts in 20 countries have publicly declared that they are abandoning FGM, including more than 2,000 communities last year. Five countries have passed national legislation criminalizing the practice.
Data also indicate widespread disapproval of the practice as the majority of people in countries where FGM data exists think it should end. This includes nearly two-thirds of boys and men.
But the overall rate of progress is not enough to keep up with population growth. If current trends continue the number of girls and women subjected to FMG will increase significantly over the next 15 years.
UNICEF, with UNFPA, co-leads the largest global programme towards the elimination of FGM. It works at every level with governments, communities, religious leaders and a multitude of other partners to end the practice.
With the inclusion of a target on eliminating FGM by 2030 in the new Sustainable Development Goals, the international community’s commitment to end FGM is stronger than ever.