- A total of 3130 new suspected cholera cases and 0 deaths were reported in Yemen for January 2017.
- The cumulative number of suspected cases of cholera reported since the start of the outbreak in October 2016 is 18 973 cases, including 99 deaths with a case-fatality rate of 0.5%. More than one third of the cases were children under the age of 5.
- Of the new cases reported, 11 stool samples were laboratory-confirmed for Vibrio Cholerae 01.
- The trend of suspected cholera cases has been declining over the past few weeks as prevention measures take hold across the country.
- In 2016, the cumulative number of suspected cholera cases was 15 843, including 531 associated deaths, with a case-fatality rate of 0.6%.
Archive for February, 2017
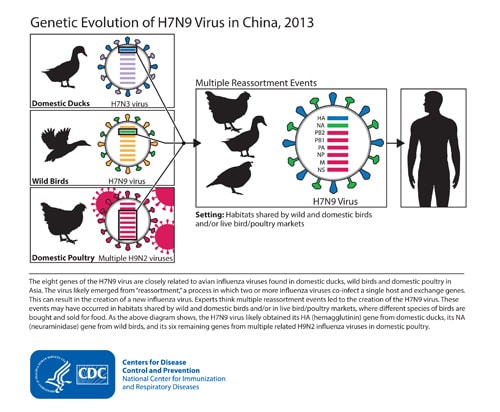
China’s Prime Minister urged local authorities to shut down live poultry markets in places affected by the H7N9 bird flu virus which killed 79 people in January.
Friday, February 24th, 2017Continued Endemic Wild Poliovirus Transmission in Nigeria, 2016
Friday, February 24th, 2017Nnadi C, Damisa E, Esapa L, et al. Continued Endemic Wild Poliovirus Transmission in Security-Compromised Areas — Nigeria, 2016. MMWR Morb Mortal Wkly Rep 2017;66:190–193. DOI: http://dx.doi.org/10.15585/mmwr.mm6607a2.

Gaza: Gazans are rebuilding after living through wars, but after so many years of isolation, residents of Gaza find themselves ever further from Palestinians in the West Bank, their future clouded by rising doubts that they could ever unite and work toward a lasting peace.
Thursday, February 23rd, 2017- Two million tons of rubble have been cleared
- Two-thirds of the 160,000 damaged homes have been rebuilt
- Half of the 11,000 homes that were destroyed have been rebuilt
- Roads are better, travel faster.
- The first real mall, with a food court and 12 escalators
- Unemployment is high, especially among the many young people graduating from college.
- 50,000 people remain displaced.
- Electricity and water supplies are still near crisis levels.
- Tunnel building goes on
https://www.youtube.com/watch?v=TNgLFgTR_rY
https://www.youtube.com/watch?v=bb8c-h_4ux4
Experimental PfSPZ malaria vaccine provides durable protection against multiple strains in NIH clinical trial
Thursday, February 23rd, 2017Tuesday, February 21, 2017
Experimental PfSPZ malaria vaccine provides durable protection against multiple strains in NIH clinical trial
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease.”
—Anthony S. Fauci, M.D., Director, NIAID
An investigational malaria vaccine has protected a small number of healthy U.S. adults from infection with a malaria strain different from that contained in the vaccine, according to a study published today in the Proceedings of the National Academy of Sciences (PNAS). The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, sponsored and co-conducted the Phase 1 clinical trial.
Malaria is transmitted to humans through the bite of infected mosquitoes, which inject immature malaria parasites called sporozoites into a person’s bloodstream. The parasites travel to the liver, where they mature, multiply and spread via the bloodstream throughout the body causing malaria symptoms including chills, fever, headache, nausea, sweating and fatigue. According to the World Health Organization, 214 million people were infected with malaria globally in 2015 and 438,000 people died, mostly young African children. The species Plasmodium falciparum is the most common cause of malaria morbidity and mortality in Africa. In the United States, travel-related malaria is a concern for international tourists, aid workers and military personnel worldwide.
The PfSPZ Vaccine used in this study was developed by Sanaria Inc., of Rockville, Maryland. The vaccine contains weakened P. falciparum sporozoites that do not cause infection but are able to generate a protective immune response against live malaria infection. Earlier research at the NIH Clinical Center with the PfSPZ Vaccine found it to be safe, well-tolerated and protective for more than a year when tested in healthy U.S. adults against a single Africa-derived malaria strain matched to the PfSPZ Vaccine.
“An effective malaria vaccine will need to protect people living in endemic areas against multiple strains of the mosquito-borne disease,” said NIAID Director Anthony S. Fauci, M.D. “These new findings showing cross-protection with the PfSPZ Vaccine suggest that it may be able to accomplish this goal.”
The study enrolled 31 healthy adults ages 18 to 45 years, and was led by Julie E. Ledgerwood, D.O. of NIAID’s Vaccine Research Center (VRC), and Kirsten E. Lyke, M.D. of the University of Maryland Center for Vaccine Development in Baltimore. Participants were assigned to receive three doses of the PfSPZ Vaccine at eight-week intervals by rapid intravenous injection.
Nineteen weeks after receiving the final dose of the test vaccine, participants who received the vaccine and a group of non-vaccinated volunteers were exposed in a controlled setting to bites from mosquitoes infected with the same strain of P. falciparum parasites (NF54, from Africa) that were used to manufacture PfSPZ Vaccine.
Nine of the 14 participants (64 percent) who received PfSPZ Vaccine demonstrated no evidence of malaria parasites; all six of the non-vaccinated participants who were challenged at the same time had malaria parasites in their blood.
Of the nine participants who showed no evidence of malaria, six participants were again exposed in a controlled setting to mosquito bites, this time from mosquitoes infected with a different strain of P. falciparum parasite, 33 weeks after the final immunization. In this group, 5 of the 6 participants (83 percent) were protected against malaria infection; none of the six participants who did not receive the vaccine and were challenged were protected. All participants who became infected with malaria immediately received medical treatment.
“Achieving durable protection against a malaria strain different from the vaccine strain, over eight months after vaccination, is an indication of this vaccine’s potential,” said Robert A. Seder, M.D., chief of the Cellular Immunology Section of NIAID’s Vaccine Research Center and senior author of the PNAS paper. “If we can build on these findings with the PfSPZ Vaccine and induce higher efficacy, we may be on our way to a vaccine that could effectively protect people against a variety of malaria parasites where the disease is prevalent.”
The research team found that the PfSPZ Vaccine activated T cells, a key component of the body’s defenses against malaria, and induced antibody responses in all vaccine recipients. Vaccine-specific T-cell responses were comparable when measured against both of the malaria challenge strains, providing some insights into how the vaccine was mediating protection.
Ongoing research will determine whether protective efficacy can be improved by changes to the PfSPZ Vaccine dose and number of immunizations. Accordingly, a Phase 2 efficacy trial testing three different dosages in a three-dose vaccine regimen is now underway in 5-to 12-month-old infants in Western Kenya to assess safety and efficacy against natural infection.
Sanaria Inc., designed, manufactured, and provided PfSPZ Vaccine and the heterologous challenge mosquitoes. NIAID supported the development of the experimental vaccine through several Small Business Innovation Research grants: 5R44AI055229-11, 5R44AI058499-08, and 5R44AI058375-08. For more information about the Phase 1 study, see clinicaltrials.gov using the identifier: NCT02015091.
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®
Reference
K. Lyke et al. PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. PNAS DOI 10.1073/pnas.1615324114 (2017).
Cholera in Yemen
Thursday, February 23rd, 2017Cholera monthly report for Yemen
AGS-v: An investigational vaccine that triggers an immune response to mosquito saliva rather than to a specific virus or parasite carried by mosquitoes
Wednesday, February 22nd, 2017The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), has launched a Phase 1 clinical trial to test an investigational vaccine intended to provide broad protection against a range of mosquito-transmitted diseases, such as Zika, malaria, West Nile fever and dengue fever, and to hinder the ability of mosquitoes to transmit such infections. The study, which is being conducted at the NIH Clinical Center in Bethesda, Maryland, will examine the experimental vaccine’s safety and ability to generate an immune response.

The investigational vaccine, called AGS-v, was developed by the London-based pharmaceutical company SEEK, which has since formed a joint venture with hVIVO in London. The consulting group Halloran has provided regulatory advice to both companies.
Unlike other vaccines targeting specific mosquito-borne diseases, the AGS-v candidate is designed to trigger an immune response to mosquito saliva rather than to a specific virus or parasite carried by mosquitoes. The test vaccine contains four synthetic proteins from mosquito salivary glands. The proteins are designed to induce antibodies in a vaccinated individual and to cause a modified allergic response that can prevent infection when a person is bitten by a disease-carrying mosquito.
“Mosquitoes cause more human disease and death than any other animal,” said NIAID Director Anthony S. Fauci, M.D. “A single vaccine capable of protecting against the scourge of mosquito-borne diseases is a novel concept that, if proven successful, would be a monumental public health advance.”
Led by Matthew J. Memoli, M.D., director of the Clinical Studies Unit in NIAID’s Laboratory of Infectious Diseases, the clinical trial is expected to enroll up to 60 healthy adults ages 18 to 50 years. Participants will be randomly assigned to receive one of three vaccine regimens. The first group will receive two injections of the AGS-v vaccine, 21 days apart. The second group will receive two injections of AGS-v combined with an adjuvant, 21 days apart. The adjuvant is an oil and water mixture commonly added to vaccines to enhance immune responses. The third group will receive two placebo injections of sterile water 21 days apart. Neither the study investigators nor the participants will know who is assigned to each group.
Participants will be asked to return to the clinic twice between vaccinations and twice after the second vaccination to undergo a physical exam and to provide blood samples. Study investigators will examine the blood samples to measure levels of antibodies triggered by vaccination.
Each participant also will return to the Clinical Center approximately 21 days after completing the vaccination schedule to undergo a controlled exposure to biting mosquitoes. The mosquitoes will not be carrying viruses or parasites, so the participants are not at risk of becoming infected with a mosquito-borne disease. Five to 10 female Aedes aegypti mosquitoes from the insectary in NIAID’s Laboratory of Malaria and Vector Research will be put in a feeding device that will be placed on each participant’s arm for 20 minutes. The mosquitoes will bite the participants’ arms through the netting on the feeding devices.
Afterward, investigators will take blood samples from each participant at various time points to see if participants experience a modified response to the mosquito bites as a result of AGS-v vaccination.
Investigators also will examine the mosquitoes after the feeding to assess any changes to their life cycle. Scientists suspect that the mosquitoes who take a blood meal from ASG-v-vaccinated participants may have altered behavior that could lead to early death or a reduced ability to reproduce. This would indicate that the experimental vaccine could also hinder disease transmission by controlling the mosquito population.
All participants will be asked to return to the clinic for follow-up visits every 60 days for five months following the mosquito feeding. A final clinic visit to assess long-term safety will take place approximately 10 months after the mosquito feeding. Throughout the trial, an independent Data and Safety Monitoring Board will review study data to evaluate participant safety and the overall conduct of the study. A medical monitor from NIAID’s Office of Clinical Research Policy and Regulatory Operations will also perform routine safety assessments.
The study is expected to be completed by summer 2018. For more information about the trial, see ClinicalTrials.gov using the trial identifier NCT03055000 (link is external).
3-Day Polio Immunzation Program: WHO, UNICEF and health authorities keeping Yemen polio-free.
Wednesday, February 22nd, 2017National polio immunization campaign launched in Yemen

WHO is working closely with UNICEF and health authorities to keep Yemen polio-freeSANA’A, 20 February 2017—A nationwide polio immunization campaign was launched today in Yemen by national health authorities with support from WHO and UNICEF, aiming to immunize 5 019 648 children under the age of 5.
More than 40 000 health workers are taking part in the 3-day campaign. In addition, religious and local council’s officials, as well as health educators are also mobilizing support for the campaign. High-risk groups, such as internally displaced persons (IDPs) and refugees, will also be reached.
“WHO is working closely with UNICEF and health authorities to keep Yemen polio-free. The threat of virus importation is serious and this campaign aims to curb any possible return of the virus to Yemen,” said Dr Nevio Zagaria, WHO Acting Representative in Yemen.
“WHO and its partners will continue to support the health authorities in increasing the vaccination coverage across Yemen.”
This is the first polio immunization campaign since April 2016. The security situation in Yemen has limited accessibility of many parts of the country, leaving many children at risk of vaccine preventable diseases.
As the nearly 2-year-old armed conflict in Yemen has been posing threats to the Expanded Programme on Immunization (EPI), WHO has supported the programme to keep polio vaccines safe through providing fuel, generators and solar-powered refrigerators to ensure the functionality of vaccine storage as well as cold chain transferring them from the war-torn areas into safer places.
“Despite huge security challenges, WHO is committed to supporting polio immunization campaigns and all activities of the EPI to maintain the polio-free status achieved by the country in 2006” said Dr Zagaria.
China: 2 more H7N9 infections in humans; Officials urge doctors to start antiviral treatment early, preferably within 48 hours of symptom onset, without waiting for influenza test results.
Wednesday, February 22nd, 2017National Health Commission on Strengthening the Medical Treatment of H7N9 Avian Influenza: “early detection, early reporting, early diagnosis, early treatment”
Drugs for Neglected Diseases initiative (DNDi): A collaborative, patients’ needs-driven, non-profit drug research and development (R&D) organization that is developing new treatments for neglected diseases.
Wednesday, February 22nd, 2017Drugs for Neglected Diseases initiative (DNDi) is a collaborative, patients’ needs-driven, non-profit drug research and development (R&D) organization that is developing new treatments for neglected patients.
DNDi is working on the following diseases:
- Leishmaniasis
- Sleeping Sickness (human African trypanosomiasis)
- Chagas disease
- Paediatric HIV
- Filarial diseases
- Mycetoma
- Hepatitis C
In 2015, DNDi handed over its Malaria portfolio.
Achievements
Working in partnership with private industry, public institutions, academia and NGOs, DNDi has built the largest ever R&D portfolio for kinetoplastid diseases.
To date, DNDi has successfully delivered, recommended and implemented:
- Two new fixed-dose combinations for Malaria (2007 & 2008) – ASAQ, ASMQ
- Better simpler treatment against Sleeping Sickness (2009) – NECT
- Cheaper more effective treatment in Africa against Visceral Leishmaniasis (VL) (2010) – SSG&PM
- A set of treatments in South Asia for VL (2011)
- A paediatric form of benznidazole for Chagas disease (2011)
- More effective treatment for children who are co-infected with HIV and TB (2016) – Superbooster Therapy
2 hospital outbreaks of MERS in Daejeon, the Republic of Korea
Wednesday, February 22nd, 2017High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea
DOI: http://dx.doi.org/10.1016/j.ijid.2017.02.008
Objectives
To explore the epidemiological and clinical factors predictive of the case fatality rate (CFR) of Middle East respiratory syndrome-coronavirus (MERS-CoV) infection in an outbreak in Daejeon, the Republic of Korea.
Methods
We reviewed the outbreak investigation reports and medical records of 1 index case and 25 additional MERS cases in hospitals A (14 cases) and B (11 cases), and conducted an in-depth interview with the index case.
Results
The CFR in hospital B was higher than that in hospital A (63.6% vs. 28.6%, respectively). Higher MERS-CoV exposure conditions were also found in hospital B, including aggravated pneumonia in the index case and nebulizer use in a six-bed admission room. The host factors associated with high CFR were pre-existing pneumonia, smoking history, an incubation period of less than 5 days, leukocytosis, abnormal renal function at diagnosis, and respiratory symptoms such as sputum and dyspnea.
Conclusions
The conditions surrounding MERS-CoV exposure and the underlying poor pulmonary function due to a smoking history or pre-existing pneumonia may explain the high CFR in hospital B. The clinical features described above may enable prediction of the prognosis of MERS cases.