Archive for November, 2017
M 4.4 – 10km ENE of Dover, Delaware
Thursday, November 30th, 2017Most of North America east of the Rocky Mountains has infrequent earthquakes. Here and there earthquakes are more numerous, for example in the New Madrid seismic zone centered on southeastern Missouri, in the Charlevoix-Kamouraska seismic zone of eastern Quebec, in New England, in the New York – Philadelphia – Wilmington urban corridor, and elsewhere. However, most of the enormous region from the Rockies to the Atlantic can go years without an earthquake large enough to be felt, and several U.S. states have never reported a damaging earthquake.
Earthquakes east of the Rocky Mountains, although less frequent than in the West, are typically felt over a much broader region than earthquakes of similar magnitude in the west. East of the Rockies, an earthquake can be felt over an area more than ten times larger than a similar magnitude earthquake on the west coast. It would not be unusual for a magnitude 4.0 earthquake in eastern or central North America to be felt by a significant percentage of the population in many communities more than 100 km (60 mi) from its source. A magnitude 5.5 earthquake in eastern or central North America might be felt by much of the population out to more than 500 km (300 mi) from its source. Earthquakes east of the Rockies that are centered in populated areas and large enough to cause damage are, similarly, likely to cause damage out to greater distances than earthquakes of the same magnitude centered in western North America.
Most earthquakes in North America east of the Rockies occur as faulting within bedrock, usually miles deep. Few earthquakes east of the Rockies, however, have been definitely linked to mapped geologic faults, in contrast to the situation at plate boundaries such as California’s San Andreas fault system, where scientists can commonly use geologic evidence to identify a fault that has produced a large earthquake and that is likely to produce large future earthquakes. Scientists who study eastern and central North America earthquakes often work from the hypothesis that modern earthquakes occur as the result of slip on preexisting faults that were formed in earlier geologic eras and that have been reactivated under the current stress conditions. The bedrock of Eastern North America is, however, laced with faults that were active in earlier geologic eras, and few of these faults are known to have been active in the current geologic era. In most areas east of the Rockies, the likelihood of future damaging earthquakes is currently estimated from the frequencies and sizes of instrumentally recorded earthquakes or earthquakes documented in historical records.
Induced Seismicity
As is the case elsewhere in the world, there is evidence that some central and eastern North America earthquakes have been triggered or caused by human activities that have altered the stress conditions in earth’s crust sufficiently to induce faulting. Activities that have induced felt earthquakes in some geologic environments have included impoundment of water behind dams, injection of fluid into the earth’s crust, extraction of fluid or gas, and removal of rock in mining or quarrying operations. In much of eastern and central North America, the number of earthquakes suspected of having been induced is much smaller than the number of natural earthquakes, but in some regions, such as the south-central states of the U.S., a significant majority of recent earthquakes are thought by many seismologists to have been human-induced. Even within areas with many human-induced earthquakes, however, the activity that seems to induce seismicity at one location may be taking place at many other locations without inducing felt earthquakes. In addition, regions with frequent induced earthquakes may also be subject to damaging earthquakes that would have occurred independently of human activity. Making a strong scientific case for a causative link between a particular human activity and a particular sequence of earthquakes typically involves special studies devoted specifically to the question. Such investigations usually address the process by which the suspected triggering activity might have significantly altered stresses in the bedrock at the earthquake source, and they commonly address the ways in which the characteristics of the suspected human-triggered earthquakes differ from the characteristics of natural earthquakes in the region.
Reports from Australia have caused mounting concern about flu season in the Northern Hemisphere, with record-high numbers of laboratory-confirmed flu notifications and outbreaks and higher-than-average numbers of hospitalizations and deaths
Thursday, November 30th, 2017Chasing Seasonal Influenza — The Need for a Universal Influenza Vaccine
November 29, 2017
DOI: 10.1056/NEJMp1714916
“…..Seasonal influenza epidemics cause 3 million to 5 million severe cases and 300,000 to 500,000 deaths globally each year…… The United States alone sees 140,000 to 710,000 influenza-related hospitalizations and 12,000 to 56,000 deaths each year, with the highest burden of disease affecting the very young, the very old, and people with coexisting medical conditions….”
“…..Another factor that may alter the effectiveness of influenza vaccines is the substrate used to produce them. In the United States, most influenza-vaccine viruses are propagated in eggs, although a small proportion are produced either in cell culture or by expressing specific viral proteins using recombinant DNA technologies. During the egg-based production process, the vaccine virus acquires amino acid changes that facilitate replication in eggs, notably changes in the hemagglutinin (HA) protein that mediates receptor binding.3 Since the influenza HA is the primary target of neutralizing antibodies, small modifications in this protein can cause antigenic changes in the virus and decrease vaccine effectiveness. Egg adaptation has been postulated to contribute to low vaccine effectiveness, particularly with influenza A (H3N2) viruses; however, the true impact is largely unknown…….”
“….the CDC estimates that influenza vaccination averted 40,000 deaths in the United States between the 2005–2006 and 2013–2014 seasons….”
Betel quid chewing & cancer
Thursday, November 30th, 2017“…..People start to enjoy the betel quid chewing starting in the
adolescence, and is associated with alcohol drinking and smoking. The
practice can lead to oral cancer.
In 2017, 67 (22 percent) out of 307 cancer patients in Taungoo public
hospital were suffering from head and neck cancer. Among them, 41 were
males and 26 were females. Males were aged in average 59.2 years
(range 36-81 years) and females were about 58.7 years old (range 19-86
years). Most of the cancers were in the oral cavity (34.3 percent),
followed by larynx (25.4 percent), oropharynx (11.9 percent),
nasopharynx (11.9 percent), hypopharynx (10.4 percent), lips (4.5
percent), and nose (1.5 percent).
As for social factors, about 20 patients (30 percent) had the habit of
betel quid chewing, 19 patients (28 percent) chewed betel quid and
smoked, and 19 patients (28 percent) chewed betel quid, smoked, and
drank alcohol…..”
Betel Quid with Tobacco (Gutka)
Betel quid is a combination of betel leaf, areca nut, and slaked lime.
In many countries, tobacco is also added, and the product is known as gutka, ghutka, or gutkha.
Other ingredients and flavorants are also added according to local preferences and customs (e.g., sweeteners, catechu, or spices such as cardamom, saffron, cloves, anise seeds, turmeric, and mustard).
Gutka is commercially available in foil packets and tins and is consumed by placing a pinch of the mixture in the mouth between the gum and cheek and gently sucking and chewing. The excess saliva produced by chewing may be swallowed or spit out.2
Use
- Betel quid and gutka use is reported to have stimulant and relaxation effects.2
- Global estimates report that up to 600 million men and women use some variety of betel quid.2
- Betel quid with or without tobacco is widely used in the Indian subcontinent (i.e., Bangladesh, India, Pakistan) as well as throughout Asia and the Pacific region (e.g., Cambodia, China, Indonesia, Malaysia, Philippines, Taiwan, Thailand).1,2,3,5
Health Effects
The following conditions have been associated with betel quid/gutka use:
Precancerous conditions
- Oral precancerous lesions, including erythroplakia (a reddened patch in the mouth) and leukoplakia (a white patch on the mucous membranes in the mouth that cannot be wiped off).2,6
- Oral submucous fibrosis (OSF), a precancerous lesion that stiffens the soft pink tissue that lines the inside of the mouth (i.e., oral mucosa).
Cancer
- Oral cancers—predominantly carcinomas of the lip, mouth, tongue, and pharynx2,6,8
- Cancer of the esophagus2
Other health effects
- Reproductive health outcomes such as increased risk of having a low birth-weight infant7
- Nicotine addiction4
References
- National Cancer Institute, Centers for Disease Control and Prevention, Stockholm Centre of Public Health. Smokeless Tobacco Fact Sheets. Third International Conference on Smokeless Tobacco; Stockholm. September 22–25, 2002 [cited 2015 Nov 27].
- World Health Organization. Betel-Quid and Areca-Nut Chewing and Some Areca-Nut-Derived Nitrosamines; Volume 85 [PDF–7.44 MB]. Lyon (France): World Health Organization, International Agency for Research on Cancer, 2004 [accessed 2015 Nov 27].
- Global Surveillance of Oral Tobacco Products: Total Nicotine, Unionised Nicotine and Tobacco-Specific N-nitrosamines. Tobacco Control 2011;20:e2, May 1, 2011 (10.1136/tc.2010.037465) [cited 2015 Nov 27].
- World Health Organization. Tobacco: Deadly in Any Form or Disguise
[PDF–2.58 MB]. Geneva: World Health Organization, 2006 [accessed 2015 Nov 27]. - Centers for Disease Control and Prevention. Use of Cigarettes and Other Tobacco Products Among Students Aged 13–15 Years—Worldwide, 1999–2005. Morbidity and Mortality Weekly Report 2006;55(20):553–6 [accessed 2015 Nov 27].
- Alert for an Epidemic of Oral Cancer Due to Use of the Betel Quid Substitutes Gutkha and Pan Masala: A Review of Agents and Causative Mechanisms. Mutagenesi 2004;19(9):251–62 [accessed 2015 Nov 27].
- Smokeless Tobacco and Health in India and South Asia. Respirology 2003;8(4):419–31 [cited 2015 Nov 27].
- Epidemiology of Betel Quid Usage [PDF–43.77 KB]. Annals Academy of Medicine Singapore 2004;33(Suppl):31S–36S [accessed 2015 Nov 27].
For Further Information
Centers for Disease Control and Prevention
National Center for Chronic Disease Prevention and Health Promotion
Office on Smoking and Health
E-mail: tobaccoinfo@cdc.gov
Phone: 1-800-CDC-INFO
Media Inquiries: Contact CDC’s Office on Smoking and Health press line at 770-488-5493.
WHO: An estimated 1 in 10 medical products circulating in low- and middle-income countries is either substandard or falsified
Thursday, November 30th, 20171 in 10 medical products in developing countries is substandard or falsified
WHO urges governments to take action
28 November 2017 | Geneva – An estimated 1 in 10 medical products circulating in low- and middle-income countries is either substandard or falsified, according to new research from WHO.
This means that people are taking medicines that fail to treat or prevent disease. Not only is this a waste of money for individuals and health systems that purchase these products, but substandard or falsified medical products can cause serious illness or even death.
“Substandard and falsified medicines particularly affect the most vulnerable communities,” says Dr Tedros Adhanom Ghebreyesus, WHO Director-General. “Imagine a mother who gives up food or other basic needs to pay for her child’s treatment, unaware that the medicines are substandard or falsified, and then that treatment causes her child to die. This is unacceptable. Countries have agreed on measures at the global level – it is time to translate them into tangible action.”
Since 2013, WHO has received 1500 reports of cases of substandard or falsified products. Of these, antimalarials and antibiotics are the most commonly reported. Most of the reports (42%) come from the WHO African Region, 21% from the WHO Region of the Americas, and 21% from the WHO European Region.
This is likely just a small fraction of the total problem and many cases may be going unreported. For example, only 8% of reports of substandard or falsified products to WHO came from the WHO Western Pacific Region, 6% from the WHO Eastern Mediterranean Region, and just 2% from the WHO South-East Asia Region.
“Many of these products, like antibiotics, are vital for people’s survival and wellbeing,” says Dr Mariângela Simão, Assistant Director-General for Access to Medicines, Vaccines and Pharmaceuticals at WHO. “Substandard or falsified medicines not only have a tragic impact on individual patients and their families, but also are a threat to antimicrobial resistance, adding to the worrying trend of medicines losing their power to treat”.
Prior to 2013, there was no global reporting of this information. Since WHO established the Global Surveillance and Monitoring System for substandard and falsified products, many countries are now active in reporting suspicious medicines, vaccines and medical devices. WHO has trained 550 regulators from 141 countries to detect and respond to this issue. As more people are trained, more cases are reported to WHO.
WHO has received reports of substandard or falsified medical products ranging from cancer treatment to contraception. They are not confined to high-value medicines or well-known brand names and are split almost evenly between generic and patented products.
In conjunction with the first report from the Global Surveillance and Monitoring System published today, WHO is publishing research that estimates a 10.5% failure rate in all medical products used in low- and middle-income countries.
This study was based on more than 100 published research papers on medicine quality surveys done in 88 low- and middle-income countries involving 48 000 samples of medicines. Lack of accurate data means that these estimates are just an indication of the scale of the problem. More research is needed to more accurately estimate the threat posed by substandard and falsified medical products.
Based on 10% estimates of substandard and falsified medicines, a modelling exercise developed by the University of Edinburgh estimates that 72 000 to 169 000 children may be dying each year from pneumonia due to substandard and falsified antibiotics. A second model done by the London School of Hygiene and Tropical Medicine estimates that 116 000 (64 000 – 158 000) additional deaths from malaria could be caused every year by substandard and falsified antimalarials in sub-Saharan Africa, with a cost of US$ 38.5 million (21.4 million – 52.4 million) to patients and health providers for further care due to failure of treatment.
Substandard medical products reach patients when the tools and technical capacity to enforce quality standards in manufacturing, supply and distribution are limited. Falsified products, on the other hand, tend to circulate where inadequate regulation and governance are compounded by unethical practice by wholesalers, distributors, retailers and health care workers. A high proportion of cases reported to WHO occur in countries with constrained access to medical products.
Modern purchasing models such as online pharmacies can easily circumvent regulatory oversight. These are especially popular in high-income countries, but more research is needed to determine the proportion and impact of sales of substandard or falsified medical products.
Globalization is making it harder to regulate medical products. Many falsifiers manufacture and print packaging in different countries, shipping components to a final destination where they are assembled and distributed. Sometimes, offshore companies and bank accounts have been used to facilitate the sale of falsified medicines.
“The bottom line is that this is a global problem,” says Dr Simão. “Countries need to assess the extent of the problem at home and cooperate regionally and globally to prevent the traffic of these products and improve detection and response.”
Note to editors
WHO is publishing two reports today:
- WHO launched its Global Surveillance and Monitoring System for substandard and falsified medicines, vaccines and in-vitro diagnostic tests in July 2013. This first report is based on data collected during the first 4 years of operation up to 30 June 2017.
- A study on the public health and socioeconomic impact of substandard or falsified medical products conducted by WHO and the Member State Mechanism.
This study is based on 100 literature reviews and two peer-reviewed models developed by the University of Edinburgh and The London School of Hygiene and Tropical Medicine. The 100 papers reviewed provide data for more than 48 000 samples of medicines from 88 countries. Because only 178 samples were taken in high-income countries, prevalence estimates of substandard or falsified medical products were limited to low- and middle-income countries.
Despite these limitations, these two reports represent the most comprehensive compilation to date of data related to substandard and falsified medical products and are a first step towards better understanding their public health and socioeconomic impact.
For more information, please contact:
Daniela Bagozzi
Senior Information/Communications Manager
Telephone: +41 22 791 1990
Mobile: +41 79 603 7281
E-mail: bagozzid@who.int
Christian Lindmeier
Communications Officer
Telephone: +41 22 791 1948
Mobile: +41 79 500 6552
E-mail: lindmeierch@who.int
WHO: Hundreds of thousands of Rohingya refugees who fled from Myanmar into Bangladesh are at risk from a possible outbreak of diphtheria
Wednesday, November 29th, 2017“…..The WHO describes diphtheria as a widespread, severe infectious disease that has the potential for epidemics, with a mortality rate of up to 10 percent…..”
Diphtheria once was a major cause of illness and death among children. The United States recorded 206,000 cases of diphtheria in 1921, resulting in 15,520 deaths. Starting in the 1920s, diphtheria rates dropped quickly due to the widespread use of vaccines. Between 2004 and 2015, 2 cases of diphtheria were recorded in the United States. However, the disease continues to cause illness globally. In 2014, 7,321 cases of diphtheria were reported worldwide to the World Health Organization, but many more cases likely go unreported.
The case-fatality rate for diphtheria has changed very little during the last 50 years. The overall case-fatality rate for diphtheria is 5%–10%, with higher death rates (up to 20%) among persons younger than 5 and older than 40 years of age. Before there was treatment for diphtheria, the disease was fatal in up to half of cases.
Clinical Features
The incubation period of diphtheria is 2–5 days (range: 1–10 days). Diphtheria can involve almost any mucous membrane. For clinical purposes, it is convenient to classify diphtheria into a number of manifestations, depending on the site of disease:
- Respiratory diphtheria
- Nasal diphtheria
- Pharyngeal and tonsillar diphtheria
- Laryngeal diphtheria
- Cutaneous diphtheria
Clinician Resources
- Checklist for Assessing a Patient with Suspected Diphtheria[2 pages](https://www.cdc.gov/diphtheria/downloads/dip-cklist-diag.pdf)
- Diphtheria Investigation—Worksheet[2 pages](https://www.cdc.gov/diphtheria/downloads/dip-wksht.pdf)
- Information for Close Contacts of a Diphtheria Patient—Worksheet[2 pages](https://www.cdc.gov/diphtheria/downloads/close-contacts.pdf)
Medical Management
After the provisional clinical diagnosis is made and appropriate cultures are obtained, persons with suspected diphtheria should be given antitoxin and antibiotics in adequate dosage and placed in isolation. Respiratory support and airway maintenance should also be administered as needed.
Diphtheria Antitoxin
In the United States, diphtheria antitoxin can be obtained from CDC on request. Learn more about diphtheria antitoxin and how to request it(https://www.cdc.gov/diphtheria/dat.html).
Antibiotics
The recommended antibiotic treatment for diphtheria is erythromycin orally or by injection (40 mg/kg/day; maximum, 2 gm/day) for 14 days, or procaine penicillin G daily, intramuscularly (300,000 units every 12 hours for those weighing 10 kg or less, and 600,000 units every 12 hours for those weighing more than 10 kg) for 14 days. Oral penicillin V 250 mg 4 times daily is given instead of injections to persons who can swallow. The disease is usually not contagious 48 hours after antibiotics are instituted. Elimination of the organism should be documented by two consecutive negative cultures after therapy is completed.
Preventive Measures
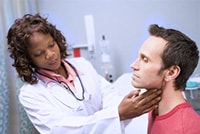
For close contacts, especially household contacts, a diphtheria toxoid booster, appropriate for age, should be given. Contacts should also receive antibiotics—a 7- to 10-day course of oral erythromycin (40 mg/kg/day for children and 1 g/day for adults). For compliance reasons, if surveillance of contacts cannot be maintained, they should receive benzathine penicillin (600,000 units for persons younger than 6 years old and 1,200,000 units for those 6 years and older). Identified carriers in the community should also receive antibiotics. Contacts should be closely monitored and antitoxin given at the first sign(s) of illness.
Contacts of cutaneous diphtheria should be treated as described above; however, if the strain is shown to be nontoxigenic, investigation of contacts can be discontinued.
Challenges
Circulation of the bacteria appears to continue in some settings, even in populations with more than 80% childhood immunization rates. An asymptomatic carrier state can exist even among immune individuals.
Immunity wanes over time and a booster dose of vaccine should be administered every 10 years to maintain protective antibody levels. Large populations of older adults may be susceptible to diphtheria, in both developed as well as in developing countries.
In countries with low disease incidence, the diagnosis may not be considered by clinicians and laboratory scientists. Prior antibiotic treatment can prevent recovery of the organism. Because the disease is rarely seen in developed countries, most physicians will never have seen a case of diphtheria in their lifetime. There is limited epidemiologic, clinical, and laboratory expertise on diphtheria.
Surveillance
National surveillance is conducted through the National Notifiable Diseases Surveillance System. Cases are also identified by requests for diphtheria antitoxin (DAT); since 1997, DAT is available for U.S. healthcare professionals only through CDC.
Need for Greater Oversight of Precursor Chemicals Sold At the Retail Level to Reduce Threats from Improvised Explosive Devices
Wednesday, November 29th, 2017Nov. 14, 2017
FOR IMMEDIATE RELEASE
New Report Calls for Greater Oversight of Precursor Chemicals Sold At the Retail Level to Reduce Threats from Improvised Explosive Devices
WASHINGTON – Policymakers’ efforts to reduce threats from improvised explosive devices (IEDs) should include greater oversight of precursor chemicals sold at the retail level – especially over the Internet – that terrorists, violent extremists, or criminals use to make homemade explosives, says a new report from the National Academies of Sciences, Engineering, and Medicine. While retail sales of these precursor chemicals present a substantial vulnerability, they have not been a major focus of federal regulations so far.
“The bombings of the Murrah Federal Building in Oklahoma City and the World Trade Center in New York City in the 1990s and those over the past few years in Paris, Brussels, and Manchester, in New York and New Jersey, and in many other communities around the world starkly demonstrate the long lived and persistent threat posed by IEDs,” said Victoria Greenfield, chair of the committee that wrote the report, and a visiting scholar in the department of criminology, law and society at George Mason University. “The report stresses the importance of engaging in an ongoing deliberative process to reduce this threat.”
The report, which was requested by the U.S. Department of Homeland Security, identifies, lists, and prioritizes precursor chemicals that can be used to make homemade explosives, using criteria on utility and use to separate them into Groups A, B, and C. The 10 chemicals in Group A constitute the greatest current threat and should be the highest priority for policymakers’ attention, the report says.
Precursor chemicals enter the supply chain as imports or through manufacturing and make their way to industrial, agricultural, and other end users. Industry tracks the movement of chemicals through much of the supply chain, but visibility and oversight appear to diminish as chemicals approach the end of the line. Data suggest that a terrorist can acquire enough precursor chemicals to manufacture homemade explosives through legal purchases from retail outlets. While the Oklahoma and New York City bombings employed thousands of pounds of precursor chemicals, many contemporary incidents have involved much smaller quantities that are readily available to consumers.
Conscious of the need to address this vulnerability yet minimize the burden on legitimate commerce, the committee assessed four general types of control strategy, directed at a subset of retail sales to noncommercial end-users, that is, the general public. Each strategy could include different combinations of mandatory and voluntary policy mechanisms, but three would feature a new control—a ban, a licensing requirement, or a registry for non-commercial purchases—and one would augment existing controls with increases in related outreach, training, and reporting.
Of the four types of strategy, none emerged as the best choice during the committee’s deliberations on security, economic, and other trade-offs, the report says, noting that the committee lacked the time, resources, and directive from DHS to do an in-depth analysis of these policy options and that such an endeavor would require greater specificity about the terms of proposed actions. The report calls on DHS to use the committee’s assessment as a starting point for engaging in a more comprehensive, detailed, and rigorous analysis of specific provisions for mandatory and voluntary policy mechanisms.
In examining possible policy options, policymakers and private-sector entities should consider strategies that would address multiple chemicals, rather than just a single chemical, the report says. Historically, terrorists’ have modified their tactics by using alternative chemicals in response to single-chemical controls. The federal government also should provide more support for voluntary measures and programs that can help restrict access. Regardless of the path chosen, the report calls for re-evaluating priorities among chemicals and re-visiting policy responses regularly, in light of changing threats.
In addition, federal, state, local, and private-sector entities should explore strategies for harmonizing oversight of the sale and use of commercially available exploding target kits that are designed to produce homemade explosives, the report says. Some states have implemented rules independently, but no federal agency has explicit authority from Congress to oversee the sale of these kits.
The committee also identified opportunities for future research, including work to identify chemicals that could replace precursor chemicals in commercial products and to better understand how terrorists might respond to possible controls.
The study was funded by the U.S. Department of Homeland Security. The National Academies of Sciences, Engineering, and Medicine are private, nonprofit institutions that provide independent, objective analysis and advice to the nation to solve complex problems and inform public policy decisions related to science, technology, and medicine. The National Academies operate under an 1863 congressional charter to the National Academy of Sciences, signed by President Lincoln. For more information, visit http://national-academies.org.
Contacts:
Riya V. Anandwala, Media Relations Officer
Joshua Blatt, Media Relations Assistant
Office of News and Public Information
202-334-2138; e-mail news@nas.edu
Social Media:
Follow us on Twitter: @theNASEM
Follow us on Instagram: @theNASEM
Follow us on Facebook: @NationalAcademies
Copies of Reducing the Threat of Improvised Explosive Device Attacks by Restricting Access to Explosive Precursor Chemicals are available at www.nap.edu or by calling 202-334-3313 or 1-800-624-6242. Reporters may obtain a copy from the Office of News and Public Information (contacts listed above).
Victoria A. Greenfield (chair)
Visiting Scholar
Department of Criminology, Law, and Society
George Mason University
Fairfax, Va.
Robert G. Best
Expert
Joint Improvised-Threat Defeat Organization
Defense Threat Reduction Agency
U.S. Department of Defense
Burke, Va.
Leo E. Bradley
Independent Consultant and Owner
LE Bradley Consulting LLC
Alexandria, Va.
John C. Brulia
Director of Safety and Compliance (retired)
Austin Powder Co.
Sagamore Hills, Ohio
Carrie L. Castille
Agriculture and Natural Resources Consultant
Breaux Bridge, La.
David G. Delaney
Senior Fellow
Center for Health and Homeland Security
University of Maryland
Baltimore
Arthur G. Fraas
Visiting Fellow
Resources for the Future
Chevy Chase, Md.
William J. Hurley
Adjunct Research Staff Member
Joint Advanced Warfighting Division
Institute for Defense Analyses
Alexandria, Va.
Karmen N. Lappo
Principal Member
Sandia National Laboratories
Albuquerque, N.M.
Becky D. Olinger
Science Adviser
Office of Counter Terrorism and Counter Proliferation
National Nuclear Security Administration
Los Alamos, N.M.
Jimmie C. Oxley
Professor of Chemistry and Co-Director
Forensic Science Partnership
University of Rhode Island
Kingston
Kevin F. Smith
President and CEO
Sustainable Supply Chain Consulting
Windermere, Fla.
Kirk Yeager
Senior Forensic Scientist
Federal Bureau of Investigation
Quantico, Va.
2017-2018 Influenza Season Week 46 ending November 18, 2017
Wednesday, November 29th, 2017Synopsis:
During week 46 (November 12-18, 2017), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 46 was influenza A. The percentage of respiratory specimens testing positive for influenza in clinical laboratories is increasing.
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Five influenza-associated pediatric deaths were reported, one of which occurred during the 2016-17 season.
- Influenza-associated Hospitalizations: A cumulative rate of 1.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 2.0%, which is below the national baseline of 2.2%. Regions 1, 2, 4 and 6 reported ILI at or above region-specific baseline levels. Two states experienced high ILI activity, one state experienced moderate ILI activity, New York City and 4 states experienced low ILI activity, the District of Columbia and 43 states experienced minimal ILI activity, and Puerto Rico had insufficient data.
- Geographic Spread of Influenza:The geographic spread of influenza in two states was reported as widespread; Guam and six states reported regional activity; 20 states reported local activity; the District of Columbia, the U.S. Virgin Islands and 21 states reported sporadic activity; one state reported no activity; and Puerto Rico did not report.
Pope Francis studiously avoided using the name of Myanmar’s persecuted Rohingya minority or directly addressing their situation
Tuesday, November 28th, 2017The two confirmed cases of Yellow Fever in Brazil (1 fatal)
Tuesday, November 28th, 2017Yellow fever – Brazil
Between July and mid-October 2017, a total of 71 suspected yellow fever cases were reported in São Paulo State, Brazil. Of these, two were confirmed, six are under investigation, and 63 were ruled out. The two confirmed cases (one of which was fatal) were reported from Itatiba from 17 September through 7 October 2017.
From July to early November, 580 epizootics in non-human primates (NHPs) were reported in São Paulo State, with an increase in the number of cases reported from 10 September 2017. Of these, 120 were confirmed for yellow fever, 233 are under investigation, 74 were classified as undetermined, and 153 were ruled out. The highest number of epizootics was registered in the health surveillance area of Campinas, where epizootic episodes were reported for the first time in the municipalities of Campo Limpo Paulista (in the week ending 23 September 2017), Atibaia (in the week ending 30 September 2017), and Jarinu (in the week ending 14 October 2017). Epizootics in NHPs were also recently reported in large parks located within the urban area of São Paulo City (in the week ending 14 October 2017).
Public health response
The detection of two confirmed yellow fever human cases and epizootics in the state of São Paulo, as well as confirmed yellow fever epizootics in the urban area of São Paulo City, prompted national authorities to begin vaccination campaigns in areas previously considered not at risk for yellow fever transmission. In addition, state and municipality health authorities are strengthening health care services and carrying out risk communication activities.
WHO risk assessment
These are the first human cases of yellow fever that have been reported in Brazil since June 2017. These cases, alongside the occurrence of epizootics in the urban area of São Paulo City and in municipalities that were previously considered not at risk for yellow fever, are a public health concern. Although Brazilian health authorities have swiftly implemented a series of public health measures in response to this event, including mass vaccination campaigns, it may take some time to reach optimal coverage in these areas given the large number of susceptible individuals. Currently, the number of unvaccinated people in São Paulo City remains high at around 10 million. If yellow fever transmission continues to spread to areas that were previously considered not at risk for yellow fever, ensuring the availability of vaccine and implementing control measures would pose significant challenges.
To date, there has been no evidence of transmission by Aedes aegypti in relation to this outbreak in Brazil which began in 2016. Although entomological studies conducted in selected municipalities of São Paulo revealed low levels of Ae. aegypti and Aedes albopictus infestation (pupa index range: 0% – 3.1%), the risk of sustained arbovirus transmission is ever present.
The risk of spread at the regional level is considered to be low given the high vaccination coverage in neighbouring countries; however, the detection of a human case of yellow fever in Oiapoque, the border river between French Guiana and Brazil in August 2017 by French health authorities indicates that the risk of regional spread exists. The risk at the global level is considered to be low and limited only to unvaccinated travellers returning from affected areas. Travelers who return home while infected with yellow fever virus may increase the risk of establishing local cycles of yellow fever transmission in areas where the competent vector is present.
WHO continues to monitor the epidemiological situation and assess the risk according to the latest available information.
WHO advice
Advice to travellers planning to visit areas at risk for yellow fever transmission in Brazil includes receiving yellow fever vaccine at least 10 days prior to traveling, following measures to avoid mosquito bites, and being aware of yellow fever symptoms and signs. WHO continues to promote health seeking behaviour when travelers are in and when they have returned from an area at risk for yellow fever transmission.
As per Annex 7 of the International Health Regulations (2005), a single dose of yellow fever vaccine is sufficient to confer sustained immunity and life-long protection against yellow fever disease. Booster doses of yellow fever vaccine are not needed.
The WHO Secretariat does not recommend any restrictions on travel or trade with/to Brazil according to the information currently available for this event.




