Archive for December, 2017
Marburg virus disease fact sheet: WHO, 2017
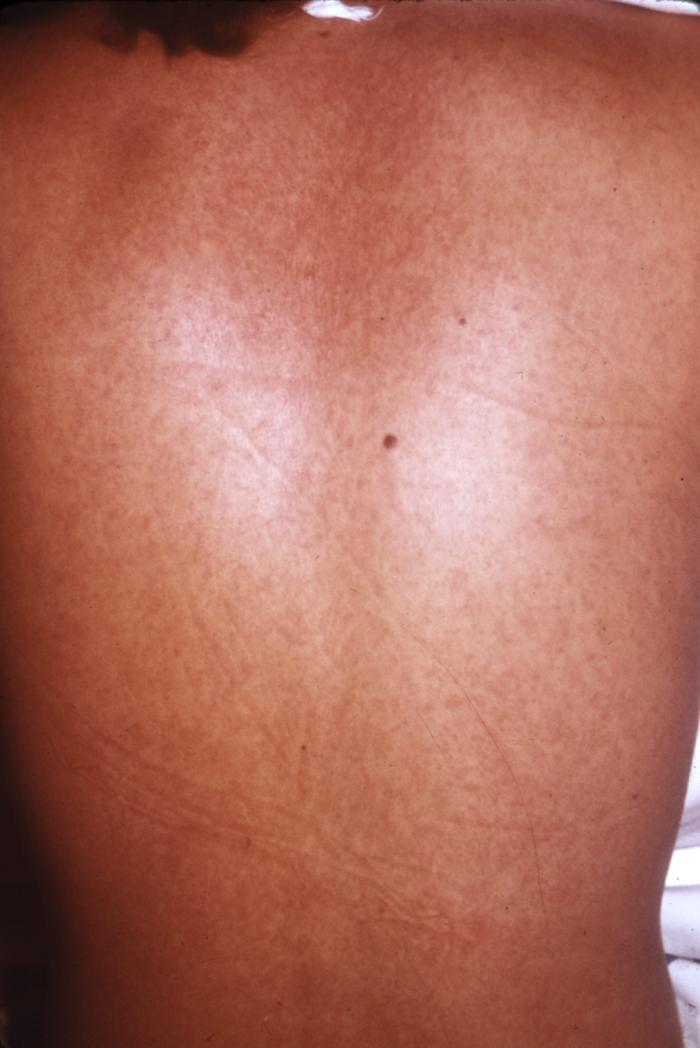
Sunday, December 10th, 2017CDC/ Conrad: This type of maculopapular rash, which can appear on Marburg patients around the fifth day after the onset of symptoms, usually may be found on the patient’s chest, back and stomach. This patient’s skin blanched under pressure, which is a common characteristic of a Marburg virus rash.
Key facts
- Marburg virus disease (MVD), formerly known as Marburg haemorrhagic fever, is a severe, often fatal illness in humans.
- Rousettus aegyptiacus, fruit bats of the Pteropodidae family, are considered to be natural hosts of Marburg virus. The Marburg virus is transmitted to people from fruit bats and spreads among humans through human-to-human transmission.
- The Marburg virus causes severe viral haemorrhagic fever in humans.
- The average MVD case fatality rate is around 50%. Case fatality rates have varied from 24% to 88% in past outbreaks depending on virus strain and case management.
- Community engagement is key to successfully controlling outbreaks. Good outbreak control relies on applying a package of interventions, namely case management, infection prevention and control practices, surveillance and contact tracing, a good laboratory service, safe burials and social mobilization.
- Early supportive care with rehydration, symptomatic treatment improves survival. There is as yet no licensed treatment proven to neutralize the virus but a range of blood products, immune therapies and drug therapies are currently under development.
Marburg virus is the causative agent of Marburg virus disease (MVD), a disease with a case fatality ratio of up to 88%. Marburg virus disease was initially detected in 1967 after simultaneous outbreaks in Marburg and Frankfurt in Germany; and in Belgrade, Serbia.
Marburg and Ebola viruses are both members of the Filoviridae family (filovirus). Though caused by different viruses, the two diseases are clinically similar. Both diseases are rare and have the capacity to cause dramatic outbreaks with high fatality rates.
Two large outbreaks that occurred simultaneously in Marburg and Frankfurt in Germany, and in Belgrade, Serbia, in 1967, led to the initial recognition of the disease. The outbreak was associated with laboratory work using African green monkeys (Cercopithecus aethiops) imported from Uganda. Subsequently, outbreaks and sporadic cases have been reported in Angola, Democratic Republic of the Congo, Kenya, South Africa (in a person with recent travel history to Zimbabwe) and Uganda. In 2008, two independent cases were reported in travelers who had visited a cave inhabited by Rousettus bat colonies in Uganda.
Transmission
Initially, human MVD infection results from prolonged exposure to mines or caves inhabited by Rousettus bat colonies.
Marburg spreads through human-to-human transmission via direct contact (through broken skin or mucous membranes) with the blood, secretions, organs or other bodily fluids of infected people, and with surfaces and materials (e.g. bedding, clothing) contaminated with these fluids.
Health-care workers have frequently been infected while treating patients with suspected or confirmed MVD. This has occurred through close contact with patients when infection control precautions are not strictly practiced. Transmission via contaminated injection equipment or through needle-stick injuries is associated with more severe disease, rapid deterioration, and, possibly, a higher fatality rate.
Burial ceremonies that involve direct contact with the body of the deceased can also contribute in the transmission of Marburg.
People remain infectious as long as their blood contains the virus.
Sexual transmission
Marburg virus transmission via infected semen has been documented up to seven weeks after clinical recovery. More surveillance data and research are needed on the risks of sexual transmission, and particularly on the prevalence of viable and transmissible virus in semen over time. In the interim, and based on present evidence, WHO recommends that:
- All Marburg survivors and their sexual partners should receive counselling to ensure safer sexual practices until their semen has twice tested negative for Marburg virus.
- Survivors should be provided with condoms.
- Male Marburg survivors should be enrolled in semen testing programmes when discharged (starting with counselling) and offered semen testing when mentally and physically ready, within three months of disease onset.
- Marburg survivors and their sexual partners should either:
- abstain from all sexual practices, or
- observe safer sexual practices through correct and consistent condom use until their semen has twice tested undetected ( negative) for Marburg virus.
- Having tested undetected (negative), survivors can safely resume normal sexual practices with minimized risk of Marburg virus transmission.
- Male survivors of Marburg virus disease should practice safer sexual practices and hygiene for 12 months from onset of symptoms or until their semen twice tests undetected (negative) for Marburg virus.
- Until such time as their semen has twice tested undetected (negative) for Marburg, survivors should practice good hand and personal hygiene by immediately and thoroughly washing with soap and water after any physical contact with semen, including after masturbation. During this period used condoms should be handled safely, and safely disposed of, so as to prevent contact with seminal fluids.
- All survivors, their partners and families should be shown respect, dignity and compassion.
Symptoms of Marburg virus disease
The incubation period (interval from infection to onset of symptoms) varies from 2 to 21 days.
Illness caused by Marburg virus begins abruptly, with high fever, severe headache and severe malaise. Muscle aches and pains are a common feature. Severe watery diarrhoea, abdominal pain and cramping, nausea and vomiting can begin on the third day. Diarrhoea can persist for a week. The appearance of patients at this phase has been described as showing “ghost-like” drawn features, deep-set eyes, expressionless faces, and extreme lethargy. In the 1967 European outbreak, non-itchy rash was a feature noted in most patients between 2 and 7 days after onset of symptoms.
Many patients develop severe haemorrhagic manifestations between 5 and 7 days, and fatal cases usually have some form of bleeding, often from multiple areas. Fresh blood in vomitus and faeces is often accompanied by bleeding from the nose, gums, and vagina. Spontaneous bleeding at venepuncture sites (where intravenous access is obtained to give fluids or obtain blood samples) can be particularly troublesome. During the severe phase of illness, patients have sustained high fevers. Involvement of the central nervous system can result in confusion, irritability, and aggression. Orchitis (inflammation of one or both testicles) has been reported occasionally in the late phase of disease (15 days).
In fatal cases, death occurs most often between 8 and 9 days after symptom onset, usually preceded by severe blood loss and shock.
Persistent virus in people recovering from Marburg virus disease
Marburg virus is known to persist in immune-privileged sites in some people who have recovered from Marburg virus disease. These sites include the testicles and the inside of the eye.
- In women who have been infected while pregnant, the virus persists in the placenta, amniotic fluid and fetus.
- In women who have been infected while breastfeeding, the virus may persist in breast milk.
Relapse-symptomatic illness in the absence of re-infection in someone who has recovered from MVD is a rare event, but has been documented. Reasons for this phenomenon are not yet fully understood.
Diagnosis
It can be difficult to clinically distinguish MVD from other infectious diseases such as malaria, typhoid fever, shigellosis, meningitis and other viral haemorrhagic fevers. Confirmation that symptoms are caused by Marburg virus infection are made using the following diagnostic methods:
- antibody-capture enzyme-linked immunosorbent assay (ELISA)
- antigen-capture detection tests
- serum neutralization test
- reverse transcriptase polymerase chain reaction (RT-PCR) assay
- electron microscopy
- virus isolation by cell culture.
Samples collected from patients are an extreme biohazard risk; laboratory testing on non-inactivated samples should be conducted under maximum biological containment conditions. All biological specimens should be packaged using the triple packaging system when transported nationally and internationally.
Treatment and vaccines
Supportive care – rehydration with oral or intravenous fluids – and treatment of specific symptoms, improves survival. There is as yet no proven treatment available for MVD. However, a range of potential treatments including blood products, immune therapies and drug therapies are currently being evaluated.
Marburg virus in animals
Rousettus aegyptiacus bats are considered natural hosts for Marburg virus. There is no apparent disease in the fruit bats. As a result, the geographic distribution of Marburg virus may overlap with the range of Rousettus bats.
African green monkeys (Cercopithecus aethiops) imported from Uganda were the source of infection for humans during the first Marburg outbreak.
Experimental inoculations in pigs with different Ebola viruses have been reported and show that pigs are susceptible to filovirus infection and shed the virus. Therefore pigs should be considered as a potential amplifier host during MHF outbreaks. Although no other domestic animals have yet been confirmed as having an association with filovirus outbreaks, as a precautionary measure they should be considered as potential amplifier hosts until proven otherwise.
Precautionary measures are needed in pig farms in Africa to avoid pigs becoming infected through contact with fruit bats. Such infection could potentially amplify the virus and cause or contribute to MHF outbreaks.
Prevention and control
Good outbreak control relies on applying a package of interventions, namely case management, surveillance and contact tracing, a good laboratory service, safe and dignified burials, and social mobilization. Community engagement is key to successfully controlling outbreaks. Raising awareness of risk factors for Marburg infection and protective measures that individuals can take is an effective way to reduce human transmission.
Risk reduction messaging should focus on several factors:
- Reducing the risk of bat-to-human transmission arising from prolonged exposure to mines or caves inhabited by fruit bat colonies. During work or research activities or tourist visits in mines or caves inhabited by fruit bat colonies, people should wear gloves and other appropriate protective clothing (including masks). During outbreaks all animal products (blood and meat) should be thoroughly cooked before consumption.
- Reducing the risk of human-to-human transmission in the community arising from direct or close contact with infected patients, particularly with their body fluids. Close physical contact with Marburg patients should be avoided. Gloves and appropriate personal protective equipment should be worn when taking care of ill patients at home. Regular hand washing should be performed after visiting sick relatives in hospital, as well as after taking care of ill patients at home.
- Communities affected by Marburg should make efforts to ensure that the population is well informed, both about the nature of the disease itself and about necessary outbreak containment measures.
- Outbreak containment measures include prompt and safe burial of the dead, identifying people who may have been in contact with someone infected with Marburg and monitoring their health for 21 days, separating the healthy from the sick to prevent further spread, and maintaining good hygiene and a clean environment need to be observed.
- Reducing the risk of possible sexual transmission. Based on further analysis of ongoing research, WHO recommends that male survivors of Marburg virus disease practice safe sex and hygiene for 12 months from onset of symptoms or until their semen twice tests negative for Marburg virus. Contact with body fluids should be avoided and washing with soap and water is recommended. WHO does not recommend isolation of male or female convalescent patients whose blood has been tested negative for Marburg virus.
Controlling infection in healthcare settings
Healthcare workers should always take standard precautions when caring for patients, regardless of their presumed diagnosis. These include basic hand hygiene, respiratory hygiene, use of personal protective equipment (to block splashes or other contact with infected materials), safe injection practices and safe and dignified burial practices.
Healthcare workers caring for patients with suspected or confirmed Marburg virus should apply extra infection control measures to prevent contact with the patient’s blood and body fluids and contaminated surfaces or materials such as clothing and bedding. When in close contact (within 1 metre) of patients with MVD, health-care workers should wear face protection (a face shield or a medical mask and goggles), a clean, non-sterile long-sleeved gown, and gloves (sterile gloves for some procedures).
Laboratory workers are also at risk. Samples taken from humans and animals for investigation of Marburg infection should be handled by trained staff and processed in suitably equipped laboratories.
WHO response
WHO aims to prevent Marburg outbreaks by maintaining surveillance for Marburg virus disease and supporting at-risk countries to develop preparedness plans. The following document provides overall guidance for control of Ebola and Marburg virus outbreaks:
When an outbreak is detected WHO responds by supporting surveillance, community engagement, case management, laboratory services, contact tracing, infection control, logistical support and training and assistance with safe burial practices.
WHO has developed detailed advice on Marburg infection prevention and control:
Table: Chronology of major Marburg virus disease outbreaks
| Year | Country | Cases | Deaths | Case fatality Rate |
| 2014 | Uganda | 1 | 1 | 100% |
| 2012 | Uganda | 15 | 4 | 27% |
| 2008 | Netherland (ex-Uganda) | 1 | 1 | 100% |
| 2008 | United States of America (ex-Uganda) | 1 | 0 | 0% |
| 2007 | Uganda | 4 | 2 | 50% |
| 2005 | Angola | 374 | 329 | 88% |
| 1998 to 2000 | Democratic Republic of the Congo | 154 | 128 | 83% |
| 1987 | Kenya | 1 | 1 | 100% |
| 1980 | Kenya | 2 | 1 | 50% |
| 1975 | South Africa | 3 | 1 | 33% |
| 1967 | Yugoslavia | 2 | 0 | 0% |
| 1967 | Germany | 29 | 7 | 24% |
Uganda ends Marburg virus disease outbreak
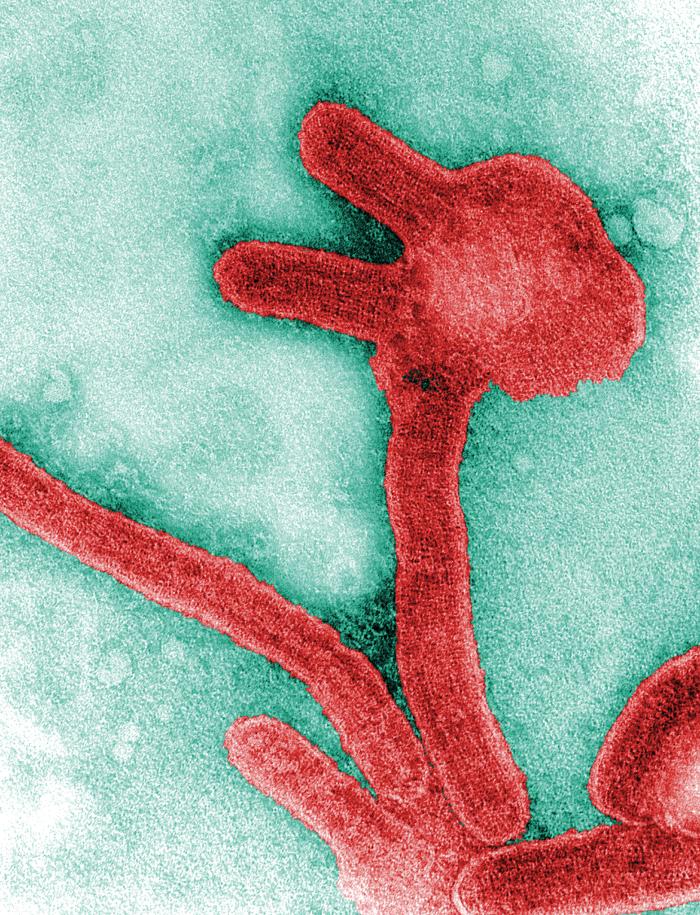
Sunday, December 10th, 2017Marburg virus virions: CDC/Murphy
8 December 2017 | Geneva – Uganda has successfully controlled an outbreak of Marburg virus disease and prevented its spread only weeks after it was first detected, the World Health Organization said on Friday (December 8).
“Uganda has led an exemplary response. Health authorities and partners, with the support of WHO, were able to detect and control the spread of Marburg virus disease within a matter of weeks,” said Dr Matshidiso Moeti, WHO Regional Director for Africa.
The Ugandan Ministry of Health notified WHO of the outbreak on October 17, after laboratory tests confirmed that the death of a 50-year-old woman was due to infection with the Marburg virus. A Public Health Emergency Operations Centre was immediately activated and a national taskforce led the response.
Three people died over the course of the outbreak which affected two districts in eastern Uganda near the Kenyan border, Kween and Kapchorwa. Health workers followed up with a total 316 close contacts of the patients in Uganda and Kenya to ensure that they had not acquired the illness.
The MVD outbreak was declared contained by the Ministry of Health after the contacts of the last confirmed patient completed 21 days of follow up (to account for the 21-day incubation period of the virus) and an additional 21 days of intensive surveillance was completed in affected districts.
“As evidenced by the quick and robust response to the Marburg virus disease outbreak, we are committed to protecting people by ensuring that all measures are in place for early detection and immediate response to all viral haemorrhagic fever outbreaks,” said Ugandan Minister of Health Sarah Opendi.
Within 24 hours of being informed by Ugandan health authorities in early October, WHO deployed a rapid response team to the remote mountainous area. The Organization also released US$623,000 from its Contingency Fund for Emergencies (CFE) to finance immediate support and scale up of the response in Uganda and Kenya.
In subsequent weeks, WHO and partners supported laboratory testing and surveillance, the search for new cases and their contacts, establishing infection prevention measures in health facilities, managing and treating cases, and engaging with communities.
Surveillance and contact tracing on the Kenyan side of the border by the Kenyan Ministry of Health and partners also prevented cross-border spread of the disease.
“The response to the Marburg virus disease outbreak demonstrates how early alert and response, community engagement, strong surveillance and coordinated efforts can stop an outbreak in its tracks before it ravages communities,” said Dr. Peter Salama, Executive Director of the WHO Health Emergencies Programme. “This was Uganda’s fifth MVD outbreak in ten years. We need to be prepared for the next one.”
WHO will continue to support health authorities in both countries to upgrade their surveillance and response capabilities – including infection prevention and control measures, and case management.
Note to editors
The response to the Marburg virus disease outbreak was led by health authorities in Uganda and Kenya in coordination with the World Health Organization (WHO), the Global Outbreak Alert and Response Network (GOARN), the US Centers for Disease Control and Prevention (CDC), the African Field Epidemiology Network (AFENET), UNICEF, Médecins Sans Frontières (MSF), the International Federation of Red Cross and Red Crescent Societies (IFRC), the International Committee of the Red Cross (ICRC), the Uganda Red Cross Society, the European Union Commission’s Civil Protection Mechanism and the Emergency Response Coordination Centre (ECHO-ERCC), the Bernhard Nocht Institute for Tropical Medicine and Marburg University in Germany, the European Union Mobile Lab Consortium and the Alliance for International Medical Action (ALIMA), the Uganda Virus Research Institute (UVRI), the Joint Mobile Emerging Diseases Intervention Clinical Capability (JMEDICC), the Infectious Diseases Institute of Makarere University (IDI), the Kenya Red Cross Society, and the Kenya Medical Research Institute (KEMRI).
Survivors of H7N9
Sunday, December 10th, 2017Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection
- Scientific Reports 7, Article number: 17275 (2017)
- doi:10.1038/s41598-017-17497-6
“…..Fifty-six influenza A (H7N9) survivors were investigated during the 2-year after discharge from the hospital. Results show interstitial change and fibrosis on pulmonary imaging remained 6 months after hospital discharge. Both ventilation and diffusion dysfunction improved, but restrictive and obstructive patterns on ventilation function test persisted throughout the follow-up period. For patients with acute respiratory distress syndrome lung functions improved faster during the first six months. Role-physical and Role-emotional domains in the 36-Item Short-Form Health Survey were worse than those of a sex- and age-matched general population group. The quality of life of survivors with ARDS was lower than those with no ARDS. Our findings suggest that pulmonary function and imaging findings improved during the first 6 months especially for those with ARDS, however long-term lung disability and psychological impairment in H7N9 survivors persisted at 2 years after discharge from the hospital…..”
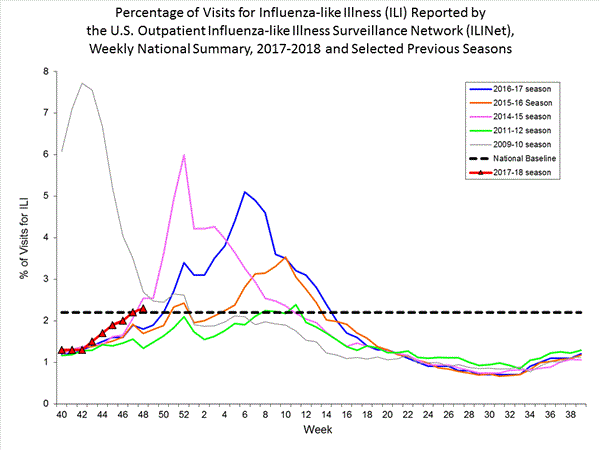
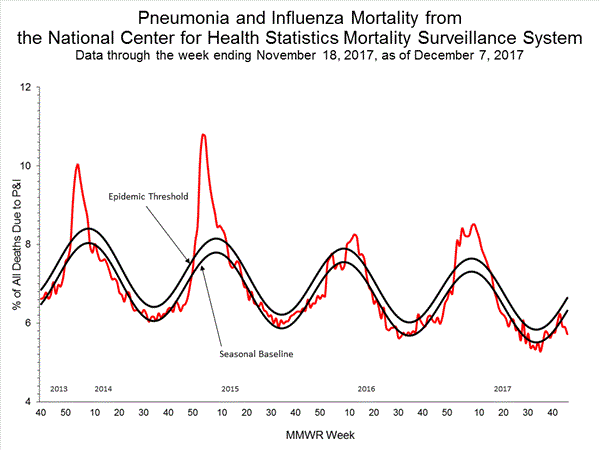
2017-2018 Influenza Season Week 48 ending December 2, 2017
Saturday, December 9th, 2017During week 48 (November 26-December 2, 2017), overall influenza activity increased slightly in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 48 was influenza A. The percentage of respiratory specimens testing positive for influenza in clinical laboratories declined slightly.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
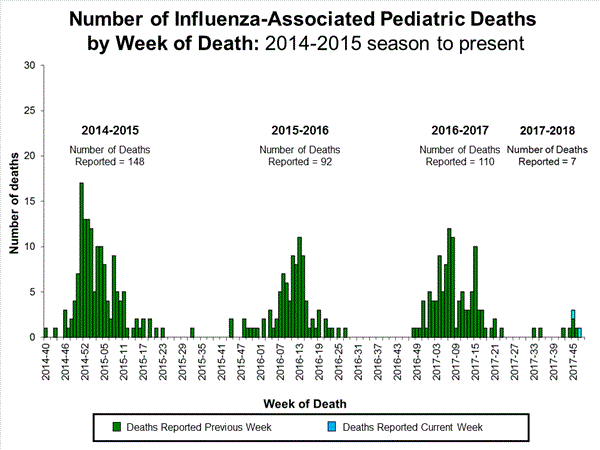
- Influenza-associated Pediatric Deaths: Two influenza-associated pediatric deaths were reported.
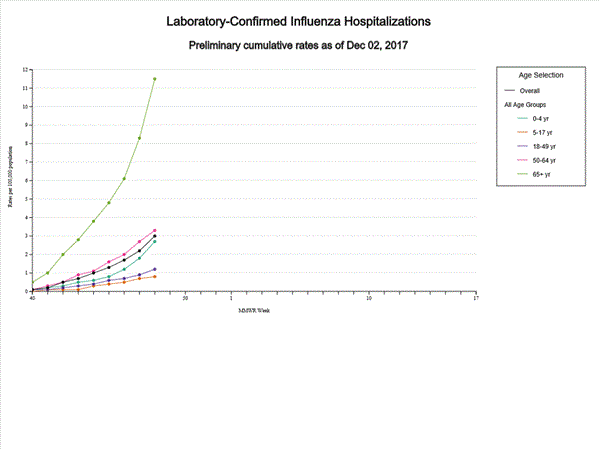
- Influenza-associated Hospitalizations: A cumulative rate of 3.0 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 2.3%, which is above the national baseline of 2.2%. Regions 1, 4, 6 and 7 reported ILI at or above region-specific baseline levels. Three states experienced high ILI activity; Puerto Rico and three states experienced moderate ILI activity; the District of Columbia and six states experienced low ILI activity; and New York City and 38 states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in seven states was reported as widespread; Puerto Rico and 18 states reported regional activity; 18 states reported local activity; and the District of Columbia, the U.S. Virgin Islands and seven states reported sporadic activity; and Guam did not report.
Puerto Rico: Official Death Toll: 62. Actual Deaths May Be 1,052.
Saturday, December 9th, 2017“…..The Times’s analysis found that in the 42 days after Hurricane Maria made landfall on Sept. 20 as a Category 4 storm, 1,052 more people than usual died across the island. The analysis compared the number of deaths for each day in 2017 with the average of the number of deaths for the same days in 2015 and 2016.…..”
Snow wreaks havoc in Northern Ireland
Friday, December 8th, 2017“…..Snow showers will continue in Northern Ireland on Friday night, with some of them heavy in the north and east.
Snow fell across many parts of NI earlier, resulting in travel disruption. A number of schools closed, as did Belfast Zoo.
Translink has said it has had to terminate some buses along parts of their routes on Friday evening.
There are also a number of delays and cancellations to flights at both the International and Belfast City Airport…..”
https://www.youtube.com/watch?v=TcYjXccLjD4
A huge swath of Southern California is now in flames.
Friday, December 8th, 2017“….as of very late Thursday night, six large fires had burned 220 square miles, 190,000 residents were evacuated, 23,000 homes were threatened, 500 were confirmed destroyed and there were 5,700 firefighters on the lines…..”
| Rye Fire: more info… | Updated: December 07, 2017 6:42 pm | |
| County: | Los Angeles County | |
| Location: | along Rye Canyon Loop, west Valencia | |
| Acres Burned – Containment: | 7,000 acres – 25% contained | |
| Evacuation Info: | 12/7/17 AM – See the latest Incident Update for more information on this fire. | |
| Creek Fire: more info… | Updated: December 07, 2017 8:28 pm | |
| County: | Los Angeles County | |
| Location: | Kagel Canyon Rd, north of Lake View terrace | |
| Acres Burned – Containment: | 15,323 acres – 20% contained | |
| Evacuation Info: | Evacuation MapSee the latest Re-population Update for more information.
12/7/17 AM – See the latest Incident Update for more information on this fire. |
|
| Thomas Fire: more info… | Updated: December 07, 2017 6:33 pm | |
| County: | Ventura County | |
| Location: | Hwy 150 and Hwy 126, north of Santa Paula | |
| Acres Burned – Containment: | 115,000 acres – 5% contained | |
| Evacuation Info: | Evacuation Map12/7/17 AM – See the latest Incident Update for more information on this fire. | |
| Lilac Fire: more info… | Updated: December 07, 2017 7:39 pm | |
| County: | San Diego County | |
| Location: | Old Hwy 395 at Dulin Road, Bonsall | |
| Acres Burned – Containment: | 4,100 acres | |
| Evacuation Info: | Mandatory Evacuations in the area of W. Lilac Rd. & Sullivan Middle School. South of Burma Rd. East of Wilshire North of N. River Rd. West of S. Mission Ave New evacuation orders: South of Renche Rd., West of I-15 Freeway, East of Green Canyon Rd. & S. Mission Rd., North of Hwy 76 Wvacuation Warnings are in effect: North of Pala Rd. South of Reche Rd. West of I-15 Freeway East of Green Canyon Rd. & W. Mission Rd. Evacuation shelters have been set up: Fallbrook High School, and Pala CasinoEast Valley Community Center located at 2245 E. Valley Parkway, Escondidio & Stagecoach Community Park located at 3420 Camino De Los Coches, Carlsbad |
|
| Liberty Fire: more info… | Updated: December 07, 2017 4:46 pm | ||||||||||||||||
| County: | Riverside County | ||||||||||||||||
| Location: | Los Alamos Rd & Liberty Rd, city of Murrieta | ||||||||||||||||
| Acres Burned – Containment: | 300 acres – 5% contained
|
||||||||||||||||
Management & Treatment: Guinea Worm Disease (GWD)
Friday, December 8th, 2017Management of Guinea Worm Disease (GWD)
When the Guinea worm is ready to come out of the body, it creates a painful burning blister on the skin. When the infected person immerses the blister in cool water to ease the symptoms, the Guinea worm breaks through the blister and part of the worm is exposed. Management of GWD involves removing the whole worm and caring for the wound in general. There is no specific drug to treat or prevent GWD. There is also no vaccine to prevent GWD. The only way to avoid infection is to prevent exposure to the Guinea worm larvae in contaminated drinking water sources[1].

Child immersing foot in water to hasten worm emergence. Photo credit: Louise Gubb, 2007, The Carter Center.
Optimal management of GWD involves the following steps[1]:
- 1. First, each day the affected body part is immersed in a container of water to encourage more of the worm to come out. To prevent contamination, the infected person is not allowed to enter drinking water sources.
-
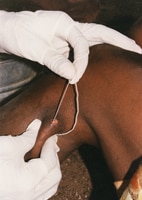
Guinea worm extraction from foot. Photo credit: Emily Staub, 2001, The Carter Center.
- 2. Next, the wound is cleaned.
- 3. Then, gentle traction is applied to the worm to slowly pull it out. Pulling stops when resistance is met to avoid breaking the worm. Because the worm can be as long as one meter in length, full extraction can take several days to weeks.
-

Guinea worm extraction from foot—worm wrapped around stick. Photo credit: Emily Staub, 2001, The Carter Center.
- 4. The worm is then wrapped around a rolled piece of gauze or a stick to maintain some tension on the worm and encourage more of the worm to emerge. This also prevents the worm from slipping back inside.
- 5. Afterwards, topical antibiotics are applied to the wound to prevent secondary bacterial infections.
-

Managing Guinea worm. Photo credit: WHO Collaborating Center at the CDC archives.
- 6. The affected body part is then bandaged with fresh gauze to protect the site. Medicines, such as aspirin or ibuprofen, are given to help ease the pain of this process and reduce inflammation.
- 7. These steps are repeated every day until the whole worm is successfully pulled out.
Progress Toward Global Eradication of Dracunculiasis, January 2016–June 2017
Friday, December 8th, 2017Donald R. Hopkins, MD1; Ernesto Ruiz-Tiben, PhD1; Mark L. Eberhard, PhD2; Sharon L. Roy, MD2; Adam J. Weiss, MPH
Dracunculiasis (Guinea worm disease) is caused by Dracunculus medinensis, a parasitic worm. Approximately 1 year after a person acquires infection from contaminated drinking water, the worm emerges through the skin, usually on a lower limb (1). Pain and secondary bacterial infection can cause temporary or permanent disability that disrupts work and schooling. The campaign to eradicate dracunculiasis worldwide began in 1980 at CDC. In 1986, the World Health Assembly called for dracunculiasis elimination,* and the global Guinea Worm Eradication Program, led by the Carter Center and supported by the World Health Organization (WHO), United Nations Children’s Fund, CDC, and other partners, began assisting ministries of health in countries with endemic dracunculiasis. In 1986, an estimated 3.5 million cases occurred each year in 20 countries in Africa and Asia (2). Since then, although the goal of eradicating dracunculiasis has not been achieved, considerable progress has been made. Compared with the 1986 estimate, the annual number of reported cases in 2016 has declined by >99%, and cases are confined to three countries with endemic disease. This report updates published (3–4) and unpublished surveillance data reported by ministries of health and describes progress toward dracunculiasis eradication during January 2016–June 2017. In 2016, a total of 25 cases were reported from three countries (Chad [16], South Sudan [six], Ethiopia [three]), compared with 22 cases reported from the same three countries and Mali in 2015 (Table 1). The 14% increase in cases from 2015 to 2016 was offset by the 25% reduction in number of countries with indigenous cases. During the first 6 months of 2017, the overall number of cases declined to eight, all in Chad, from 10 cases in three countries (Chad [four], South Sudan [four] and Ethiopia [two]) during the same period of 2016. Continued active surveillance, aggressive detection, and appropriate management of cases are essential eradication program components; however, epidemiologic challenges, civil unrest, and insecurity pose potential barriers to eradication.
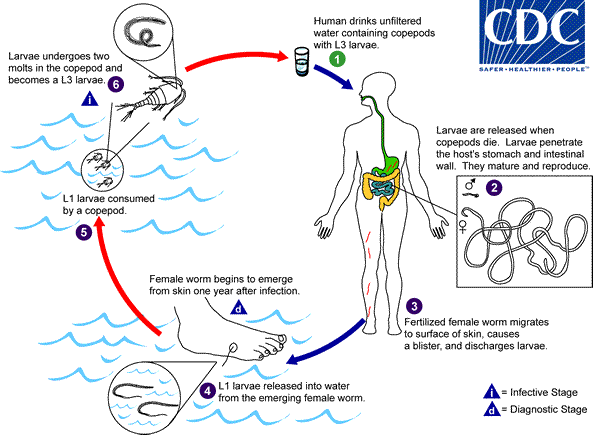
Because the life cycle of D. medinensis is complex, its transmission can be interrupted using multiple strategies (1). Dracunculiasis can be prevented by the following four main interventions: 1) educating residents in communities where the disease is endemic, particularly persons from whom worms are emerging, to avoid immersing affected body parts in sources of drinking water; 2) filtering potentially contaminated drinking water through a cloth filter or pipe filters to remove copepods (small crustaceans that host D. medinensis larvae); 3) treating potentially contaminated surface water with the organophosphate insecticide temephos (Abate) to kill the copepods; and 4) providing safe drinking water from bore-hole or protected hand-dug wells (5). Containment† of transmission is achieved through four complementary measures: 1) voluntary isolation and education of each patient to prevent contamination of drinking water sources, 2) provision of first aid to prevent secondary infections, 3) manual extraction of the worm, and 4) application of occlusive bandages. No vaccine or medicine to prevent or treat Guinea worm disease currently exists.
D. medinensis has an approximately 1-year incubation period (range = 10–14 months) after infection (5). A case of dracunculiasis is defined as an infection occurring in a person exhibiting a skin lesion or lesions with emergence of one or more worms that are laboratory-confirmed at CDC as D. medinensis. Each infected person is counted as a case only once during a calendar year. Because certain patients have multiple Guinea worms emerge, more laboratory-confirmed specimens than cases might be reported in any given period.
Countries enter the WHO precertification stage of eradication after 1 full year with no reported indigenous§ cases. An imported case is an infection resulting from ingestion of contaminated water from a source, identified through patient interviews and epidemiologic investigation, in a place other than in the community where the patient is detected and the case reported (i.e., another country or village within the same country). Since 2012, no known internationally imported cases have been reported.
In each affected country, a national dracunculiasis eradication program receives monthly reports regarding cases from each village under active surveillance. Reporting rates are calculated as the proportion of all villages under active surveillance reporting monthly (Table 2). Active surveillance is conducted in all villages with endemic dracunculiasis or that are at high risk for importation, with daily searches of households for persons with signs or symptoms of dracunculiasis, to ensure case detection within 24 hours of worm emergence and prompt patient management to prevent contamination of water sources. Villages where endemic transmission of dracunculiasis is interrupted (i.e., zero cases reported for ≥12 consecutive months) are kept under active surveillance for 3 consecutive years. WHO certifies a country free from dracunculiasis after that country maintains adequate nationwide surveillance for ≥3 consecutive years and demonstrates that no indigenous cases occurred during that period. As of January 2016, WHO had certified 198 countries, areas, and territories as free from dracunculiasis (3). Eight countries remain to be certified: four where dracunculiasis is currently endemic (Chad, Ethiopia, Mali, and South Sudan), two in the precertification stage (Kenya and Sudan), and two never known to have had endemic dracunculiasis since the global eradication program began in 1980 (Angola and the Democratic Republic of the Congo), which are in the process of completing the requirements for certification.
During January 2016–June 2017, CDC evaluated 118 worm specimens that emerged from humans, including 108 (91.5%) from the four countries with endemic dracunculiasis, two (1.7%) from Kenya, three (2.5%) from Benin (Kenya and Benin formerly had endemic dracunculiasis), four (3.4%) from the Democratic Republic of Congo, and one (1%) from Niger. Among the 118 human worm specimens submitted, 89 (75%) were from 2016 (37 [42%] were identified as D. medinensis) and 29 were from January to June 2017 (eight [28%] were identified as D. medinensis).
During 2016, 46 animal worm specimens were submitted, and 32 (70%) were identified as D. medinensis. The 32 Dracunculus specimens came from two baboons and 13 dogs from Ethiopia, 11 dogs from Mali, and five dogs and one domestic cat from Chad. During January–June 2017, 18 animal worm specimens were submitted and 14 were identified as D. medinensis. The 114 Dracunculus specimens came from four baboons and nine dogs from Ethiopia, and one dog from Chad.
Country Reports
Chad. After a decade with no reported cases, Chad reported 10 indigenous cases in 2010. After indigenous cases were confirmed during 3 consecutive years, dracunculiasis was declared to be endemic in 2012 (6,7). In 2016, Chad reported 16 cases (nine contained) in 12 villages, compared with nine cases (none contained) in 2015. During the first half of 2017, eight cases (six contained) were reported in eight villages. One of 12 villages that reported a case in 2016, and one of eight reporting a case during January–June 2017, had reported a case previously.
In 2012, Guinea worm infections were first reported in domestic dogs in Chad (6), and since then, more dogs than humans have been identified with emerging Guinea worms. This substantial number of nonhuman infections has not occurred in any other country during the eradication campaign. Worm specimens obtained from dogs were determined to be genetically indistinguishable from D. medinensis worms removed from humans in Chad (6). A majority of infections during the current outbreak have occurred in communities along the Chari River. The Carter Center has assisted the ministry of health in implementing active village-based surveillance for the disease in approximately 1,700 villages in the at-risk zone. The working hypothesis, on the basis of biologic, environmental, and epidemiologic investigations by CDC and the Carter Center, is that the cases in humans and infected dogs are associated with the domestic and commercial fishing industry along the Chari River and involve fish, frogs, or other aquatic hosts that serve as paratenic hosts (intermediate hosts in which no development of the parasite occurs). New infections are thought to occur when humans consume inadequately cooked paratenic hosts and when such hosts are consumed raw by dogs (6). Overall, 1,011 infected dogs (and 11 infected domestic cats) were reported during 2016, which was twice the number of infected dogs (503) reported in 2015. However, during January–June 2017, 537 infected dogs were reported, which is an 18% decrease from the 653 reported during the same period of 2016. This is the first such half-yearly reduction since infected dogs were first reported in 2012, and it reflects consecutive months of declining dog infections that began in November 2016 (3).
Beginning in October 2013, Chad’s Guinea Worm Eradication Program urged villagers to cook their fish well, bury fish entrails, and prevent dogs from eating fish entrails. By June 2017, according to monthly sample surveys, this intervention was being implemented by approximately 81% of respondents in surveyed communities with populations at risk. In February 2014, health education measures began to persuade villagers to tether infected dogs until the worms emerged, to prevent contamination of water and infection of copepods. In February 2015, the program introduced a reward equivalent to US$20 for reporting and tethering an infected dog. Whereas 40%, 68%, and 68% of infected dogs were tethered in 2014, 2015, and 2016, respectively, 78% of 537 infected dogs reported during January–June 2017 were tethered.
Beginning before 2010, Chad has offered a cash reward equivalent to US$100 for reporting a human case of dracunculiasis. In areas under active surveillance, 69% of 383 residents surveyed during January–June 2017 knew of the cash reward for reporting a case of dracunculiasis, and 60% of 363 persons surveyed knew of the cash reward for reporting and tethering an infected dog.
As of June 2017, 68% of villages with endemic dracunculiasis had safe water (i.e., water sources free of copepods, such as rapidly flowing rivers, protected hand dug wells, and borehole wells). Temephos use is limited by the extremely large lagoons used for fishing and as sources of drinking water. Starting in August 2014, an innovative technique of applying temephos to smaller cordoned sections of the lagoons at entry points used by infected humans or dogs was introduced and used to protect 19, 29, 61, and 51 villages in 2014, 2015, 2016, and January–June 2017, respectively.
The Carter Center and WHO Collaborating Center for Dracunculiasis Eradication at CDC are supporting research to better understand the unusual epidemiology of the current outbreak of dracunculiasis in Chad, assess antihelminthic treatment of dogs to prevent maturation of worms, and study the food sources and movements of dogs in an area of Chad with endemic disease. In collaboration with researchers from the University of Georgia, this initiative has demonstrated that D. medinensis can use an amphibian (frog) (8) as a paratenic host in the laboratory (8) and has recovered, for the first time, a Dracunculus larva from a frog captured in the wild in Chad (9).
Ethiopia. In 2016, Ethiopia reported three cases of dracunculiasis (two contained), in two villages of the Gog district and one village of the Lare district of Gambella Region. This is the same number of cases that Ethiopia reported in 2015 and in 2014. (The origin of the third case, which was reported in September 2016, is unclear, because the patient entered Ethiopia from South Sudan approximately 1 year before emergence of his Guinea worm). Ethiopia also reported 14 infected domestic dogs and two infected baboons in 2016, compared with 13 infected dogs and one infected baboon in 2015, all in the same area of the Gog district. During January–June 2017, Ethiopia reported no human case, eight infected dogs, and four infected baboons. However, in the same area of the Gog district, there were two cases in humans, two infected dogs, and no infected baboons during the same period of 2016. The program applied temephos monthly to almost all water sources known to have been used by humans in the at-risk area throughout 2015, increased coverage threefold to include numerous smaller water sources in 2016, and is addressing additional gaps in identification of water sources related to a particular stream in the at-risk area in 2017. A cash reward, equivalent to US$20 for reporting an infected animal was introduced, and the ministry of health held three press conferences to publicize the eradication effort during 2016. There are 152 villages under active surveillance in three districts of Gambella Region. Ethiopia offers a cash reward equivalent to US$100 for reporting a case of dracunculiasis. Among 11,712 persons surveyed in the Gog district during January–June 2017, 82% were aware of the reward for reporting an infected person; 61% of 2,123 surveyed knew of the reward for reporting an infected animal.
Mali. In 2016, Mali reported no human cases of dracunculiasis for the first time since its eradication program began, compared with five cases reported from three villages in 2015. Mali reported one infected dog for the first time in 2015 and 11 infected dogs (eight contained) in 2016. Two infected dogs (one contained) were reported during January–June 2017, compared with one dog (contained), during the same period of 2016. All infected dogs were detected in the Tominian district of Segou Region, but many had been imported from other areas of Segou or adjacent Mopti Region, in which certain areas were inaccessible to the program because of insecurity. Mali has 455 villages under active surveillance. Mali offers a cash reward equivalent to US$100 for reporting a case of dracunculiasis and US$20 for reporting and tethering an infected dog. In areas under active surveillance, 79% of 23,943 persons surveyed in 2016 were aware of the cash reward for reporting a case. During January–June 2017, 80% of 2,190 persons surveyed were aware of the reward for reporting a case, and 88% of 819 persons surveyed were aware of the reward for reporting and tethering an infected dog.
South Sudan. South Sudan reported six cases of dracunculiasis (three contained) from four villages in 2016, all west of the Nile, compared with five cases (three contained) reported from five villages in 2015. The country reported no infected dogs in 2016, compared with one in 2015, which has been the only infected dog found in South Sudan to date. South Sudan has reported no cases during January–June 2017, compared with four cases (three contained) reported during the same period of 2016. South Sudan has 3,860 villages under active surveillance. In April 2014, South Sudan began offering a cash reward equivalent to approximately US$125 for reporting a case of dracunculiasis (10). The overall level of reward awareness among 495 persons queried in active surveillance areas in 2016 was 76%. In March 2017, the ministry of health doubled its cash reward for reporting a case of dracunculiasis to 10,000 South Sudanese pounds (approximately US$139) to adjust for inflation and introduced a cash reward (approximately US$20) for reporting and tethering an infected animal. Coverage with interventions in villages with endemic disease remains high (except for provision of safe sources of drinking water) (Table 2), despite increased insecurity having forced the evacuation of most expatriate staff members assisting the South Sudan Guinea Worm Eradication Program since early July 2016.
Discussion
The number of countries reporting endemic dracunculiasis decreased from four in 2015 to three in 2016, to only one country (Chad), which reported eight cases during January–June 2017. This compares with 10 cases reported by Chad, Ethiopia, and South Sudan during January–June 2016 and indicates that the goal of complete eradication of the disease is closer. The decrease in the number of infected dogs in Chad for the first time during January–June 2017 is another favorable milestone. Led by their ministers of health, Mali and Chad have launched enhanced national communication campaigns in March and July 2017, respectively, to increase awareness of rewards for reporting cases and knowledge of prevention messages; South Sudan and Ethiopia plan to launch similar campaigns later in 2017.
Political support for Guinea worm eradication remains strong in South Sudan and has improved recently in Chad, Ethiopia, and Mali. The health ministers of all four countries attended or were represented at the annual informal meeting of countries with current or former endemic dracunculiasis at the World Health Assembly in Geneva, Switzerland, in May 2016, and at the International Review Meeting for Guinea Worm Eradication Program Managers held at The Carter Center in March 2016. Mali’s Minister of Health visited an area with endemic disease in June 2016, whereas South Sudan’s Minster of Health and the regional Vice President of Gambella, Ethiopia, visited such areas in their countries in September 2016. In June 2017, Chad’s National Assembly convened a special session for a briefing on that country’s Guinea Worm Eradication Program. Insecurity remains a serious challenge to program activities, especially in Mali and South Sudan.
Additional interventions, including increased use of temephos and trials of potential anthelminthic treatments for infected dogs are beginning or underway in Chad. In addition, scientists are researching aspects of the parasite’s biology, life cycle, and molecular composition, and dog ecology. Furthermore, a case-control study of humans and dogs in households with and without infected dogs is planned in Chad for early 2018.
* http://www.who.int/neglected_diseases/mediacentre/WHA_39.21_Eng.pdf.
† Transmission from a patient with dracunculiasis is contained only if all of the following conditions are met for each emerged worm: 1) the infected patient is identified ≤24 hours after worm emergence; 2) the patient has not entered any water source because the worm emerged; 3) a village volunteer or other health care provider has managed the patient properly, by cleaning and bandaging the lesion until the worm has been fully removed manually and by providing health education to discourage the patient from contaminating any water source (if two or more emerging worms are present, transmission is not contained until the last worm is removed); 4) the containment process, including verification of dracunculiasis, is validated by a Guinea Worm Eradication Program supervisor within 7 days of emergence of the worm; and 5) temephos is used to treat potentially contaminated surface water if any uncertainty about contamination of these sources of drinking water exists, or if such a source of drinking water is known to have been contaminated.
§ An indigenous case of dracunculiasis is defined as an infection consisting of a skin lesion or lesions with emergence of one or more Guinea worms in a person who had no history of travel outside their residential locality during the preceding year.
References
- Hopkins DR, Ruiz-Tiben E, Eberhard ML, Roy SL, Weiss AJ. Progress toward global eradication of dracunculiasis—January 2015–June 2016. MMWR Morb Mortal Wkly Rep 2016;65:1112–6. CrossRef
- Watts SJ. Dracunculiasis in Africa in 1986: its geographic extent, incidence, and at-risk population. Am J Trop Med Hyg 1987;37:119–25. CrossRef PubMed
- World Health Organization. Dracunculiasis eradication: global surveillance summary, 2016. Wkly Epidemiol Rec 2017;92:269–86. PubMed
- Hopkins DR, Ruiz-Tiben E, Weiss A, Withers PC Jr, Eberhard ML, Roy SL. Dracunculiasis eradication: and now, South Sudan. Am J Trop Med Hyg 2013;89:5–10. CrossRef PubMed
- Ruiz-Tiben E, Hopkins DR. Dracunculiasis (Guinea worm disease) eradication. Adv Parasitol 2006;61:275–309. CrossRef PubMed
- Eberhard ML, Ruiz-Tiben E, Hopkins DR, et al. The peculiar epidemiology of dracunculiasis in Chad. Am J Trop Med Hyg 2014;90:61–70. CrossRef PubMed
- CDC. Renewed transmission of dracunculiasis—Chad, 2010. MMWR Morb Mortal Wkly Rep 2011;60:744–8. PubMed
- Eberhard ML, Yabsley MJ, Zirimwabagabo H, et al. Possible role of fish and frogs as paratenic hosts of Dracunculus medinensis, Chad. Emerg Infect Dis 2016;22:1428–30. CrossRef PubMed
- Eberhard ML, Cleveland CA, Zirimwabagabo H, Yabsley MJ, Ouakou PT, Ruiz-Tiben E. Guinea worm (Dracunculus medinensis) infection in a wild-caught frog, Chad. Emerg Infect Dis 2016;22:1961–2. CrossRef PubMed
- World Health Organization. Meeting of the International Task Force for Disease Eradication, April 2015. Wkly Epidemiol Rec 2015;90:384–92. PubMed
 TABLE 1. Number of reported indigenous human dracunculiasis cases, by country –– worldwide, January 2015–June 2017
TABLE 1. Number of reported indigenous human dracunculiasis cases, by country –– worldwide, January 2015–June 2017
| Country | Cases by period | |||||
|---|---|---|---|---|---|---|
| Jan–Dec 2015 | Jan–Dec 2016 | % Change Jan–Dec 2015 to Jan–Dec 2016 | Jan–Jun, 2016* | Jan–Jun, 2017 | % Change Jan–Jun 2016 to Jan–Jun 2017 | |
| No. | No. (% contained) | No. | No. (% contained) | |||
| Chad | 9 | 16 (56) | 78 | 4 | 8 (75) | 100 |
| Ethiopia | 3 | 3 (67) | 0 | 2 | 0 (—) | –100 |
| Mali† | 5 | 0 (—) | –100 | 0 | 0 (—) | 0 |
| South Sudan | 5 | 6 (50) | 20 | 4 | 0 (—) | –100 |
| Total | 22 | 25 (56) | 14 | 10 | 8 (75) | -20 |
* No international importations were reported during the 18-month period January 2016–June 2017.
† Civil unrest and insecurity continued to constrain program operations in regions with endemic dracunculiasis (Gao, Kidal, Mopti, and Timbuktu) during 2016–2017.
 TABLE 2. Reported human dracunculiasis cases, surveillance, and status of local interventions in villages with endemic disease, by country –– worldwide, 2016
TABLE 2. Reported human dracunculiasis cases, surveillance, and status of local interventions in villages with endemic disease, by country –– worldwide, 2016
| Cases/Surveillance/Intervention status | Country | ||||
|---|---|---|---|---|---|
| Chad* | Ethiopia | Mali† | South Sudan | Total | |
| Reported cases | |||||
| No. indigenous, 2016 | 16 | 3 | 0 | 6 | 25 |
| No. imported,§ 2016 | 0 | 0 | 0 | 0 | 0 |
| % Contained¶ in 2016 | 56 | 67 | 0 | 50 | 56 |
| % Change in indigenous cases in villages/localities under surveillance, same period 2015 and 2016 | 78 | 0 | –100 | 20 | 14 |
| Villages under active surveillance, 2016 | |||||
| No. of villages | 1,799 | 152 | 450 | 2,736 | 5,137 |
| % Reporting monthly | 100 | 89 | 100 | 99 | 99 |
| No. reporting ≥1 case | 8 | 3 | 0 | 5 | 16 |
| No. reporting only imported** cases | 0 | 0 | 0 | 0 | 0 |
| No. reporting indigenous cases | 8 | 3 | 0 | 5 | 16 |
| Status of interventions in villages with endemic dracunculiasis, 2015–2016 | |||||
| No. of villages with endemic dracunculiasis | 20 | 5 | 3 | 9 | 37 |
| % Reporting monthly†† | 100 | 100 | 100 | 100 | 100 |
| % Filters in all households†† | 100 | 100 | 100 | 100 | 100 |
| % Using temephos†† | 30 | 100 | 100 | 100 | 60 |
| % ≥1 safe water source†† | 73 | 100 | 66 | 56 | 62 |
| % Providing health education†† | 100 | 100 | 100 | 100 | 100 |
* Participants at the annual Chad Guinea Worm Eradication Program review meeting in November 2014 adopted “1+ case village” as a new description for villages in Chad affected by human cases of Guinea worm disease or dogs infected with Guinea worms and defined as “a village with one or more indigenous or imported cases of Guinea worm infections in humans, dogs, or cats in the current calendar year or previous year.”
† Civil unrest and insecurity continued to constrain Guinea Worm Eradication Program operations (supervision, surveillance, and interventions in Gao, Kidal, and Timbuktu regions).
§ Imported from another country.
¶ Transmission from a patient with dracunculiasis is contained only if all of the following conditions are met for each emerged worm: 1) the infected patient is identified ≤24 hours after worm emergence; 2) the patient has not entered any water source because the worm emerged; 3) a village volunteer or other health care provider has managed the patient properly, by cleaning and bandaging the lesion until the worm has been fully removed manually and by providing health education to discourage the patient from contaminating any water source (if two or more emerging worms are present, transmission is not contained until the last worm is removed); 4) the containment process, including verification of dracunculiasis, is validated by a Guinea Worm Eradication Program supervisor within 7 days of emergence of the worm; and 5) temephos is used to treat potentially contaminated surface water if any uncertainty about contamination of these sources of drinking water exists, or if a such a source of drinking water is known to have been contaminated.
** Imported from another in-country village with endemic disease.
†† The denominator is the number of endemic villages/localities where the program applied interventions during 2015–2016.
Suggested citation for this article: Hopkins DR, Ruiz-Tiben E, Eberhard ML, Roy SL, Weiss AJ. Progress Toward Global Eradication of Dracunculiasis, January 2016–June 2017. MMWR Morb Mortal Wkly Rep 2017;66:1327–1331. DOI: http://dx.doi.org/10.15585/mmwr.mm6648a3.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.