Archive for April, 2018
A bus en route to Nipawin, foreground, carrying the Humboldt Broncos junior hockey team crashed into a truck Friday night, killing 14 and sending over a dozen more to the hospital.
Sunday, April 8th, 2018Rescuers and medics say at least 70 people have died in Syria in a suspected gas attack in Douma
Sunday, April 8th, 2018Muenster, Germany: A vehicle crashed into a crowd Saturday, killing several injuring as many as 20 more.
Saturday, April 7th, 2018https://www.youtube.com/watch?v=EfKY0fOYv60
April 7, 1994: The Rwanda Genocide begins
Saturday, April 7th, 2018“….Rwandan armed forces kill 10 Belgian peacekeeping officers in a successful effort to discourage international intervention in their genocide that had begun only hours earlier. In less than three months, Hutu extremists who controlled Rwanda murdered an estimated 800,000 innocent civilian Tutsis in the worst episode of genocide since World War II. The Tutsis, a minority group that made up about 10 percent of Rwanda’s population, received no assistance from the international community, although the United Nations later conceded that a mere 5,000 soldiers deployed at the outset would have stopped the wholesale slaughter.….”
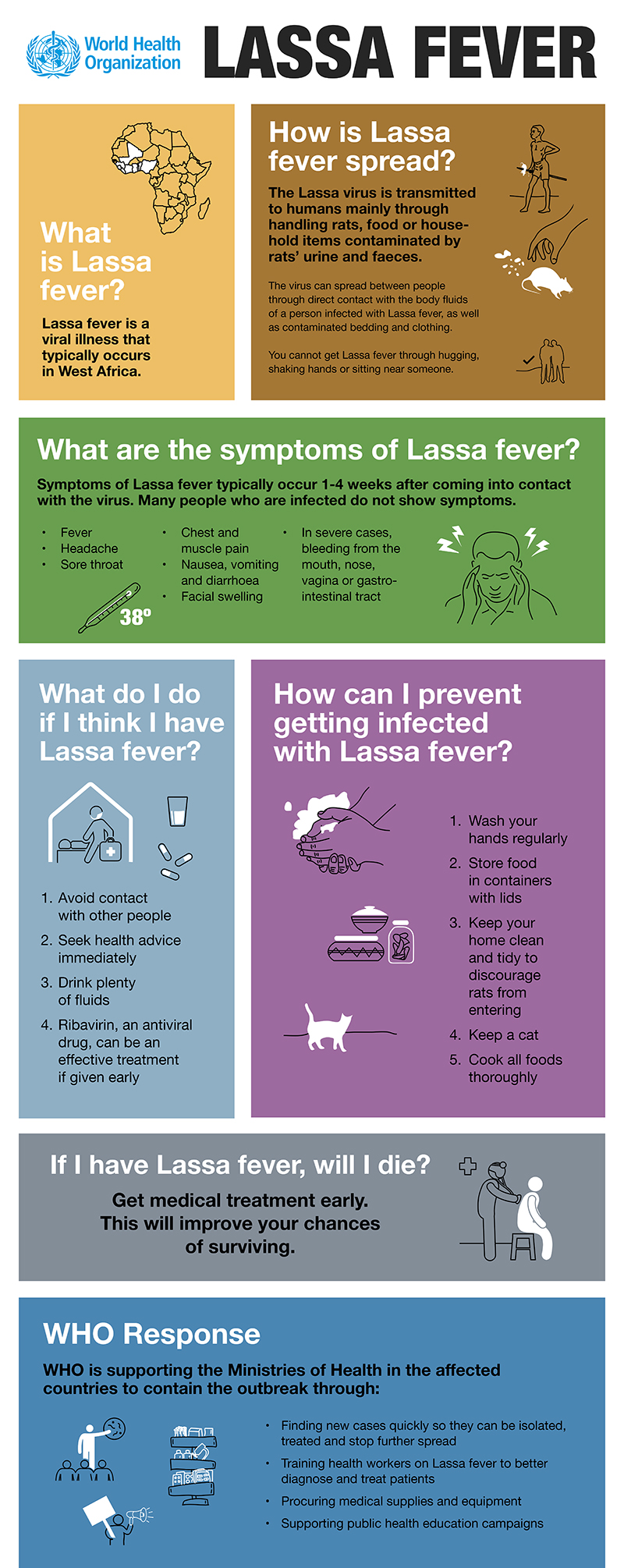
CDC has now confirmed 132 cases in 32 states tied to the use of kratom, an herbal alternative to opioids.
Saturday, April 7th, 2018What’s New?
- Forty-five more ill people from 19 states were added to this investigation since the last update on March 15, 2018.
- Three additional states have reported ill people: Connecticut, Iowa, and Idaho.
Highlights
- At this time, CDC recommends that people not consume any brand of kratom in any form because it could be contaminated with Salmonella.
- Kratom products from several companies have been recalled because they might be contaminated with Salmonella. The list of recalled kratom products is available on the U.S. Food and Drug Administration website.
- Kratom is also known as Thang, Kakuam, Thom, Ketom, and Biak.
- Kratom is a plant consumed for its stimulant effects and as an opioid substitute.
- CDC, public health and regulatory officials in several states, and the U.S. Food and Drug Administration are investigating a multistate outbreak of Salmonella infections.
- Epidemiologic and laboratory evidence indicates that kratom is the likely source of this multistate outbreak.
- No common brands or suppliers of kratom products have been identified at this time.
- Because no common source of Salmonella-contaminated kratom has been identified, CDC is recommending against consuming any kratom.
- A total of 132 people infected with outbreak strains of Salmonella I 4,[5],12:b:- (61), Salmonella Javiana (15), Salmonella Okatie (21), or Salmonella Thompson (35) have been reported from 38 states.
- Forty percent of ill people have been hospitalized, and no deaths have been reported.
- This investigation is ongoing. CDC will provide updates when more information is available.
Royal Canadian Mounted Police: A crash between a transport truck and a bus carrying a junior hockey team in the province of Saskatchewan has left 14 people dead and 14 injured.
Saturday, April 7th, 2018Lassa Fever: A WHO Review
Saturday, April 7th, 2018Key Facts
- Lassa fever is an acute viral haemorrhagic illness of 2-21 days duration that occurs in West Africa.
- The Lassa virus is transmitted to humans via contact with food or household items contaminated with rodent urine or faeces.
- Person-to-person infections and laboratory transmission can also occur, particularly in hospitals lacking adequate infection prevention and control measures.
- Lassa fever is known to be endemic in Benin, Ghana, Guinea, Liberia, Mali, Sierra Leone, and Nigeria, but probably exists in other West African countries as well.
- The overall case-fatality rate is 1%. Observed case-fatality rate among patients hospitalized with severe cases of Lassa fever is 15%.
- Early supportive care with rehydration and symptomatic treatment improves survival.
Though first described in the 1950s, the virus causing Lassa disease was not identified until 1969. The virus is a single-stranded RNA virus belonging to the virus family Arenaviridae.
About 80% of people who become infected with Lassa virus have no symptoms. 1 in 5 infections result in severe disease, where the virus affects several organs such as the liver, spleen and kidneys.
Lassa fever is a zoonotic disease, meaning that humans become infected from contact with infected animals. The animal reservoir, or host, of Lassa virus is a rodent of the genus Mastomys, commonly known as the “multimammate rat.” Mastomys rats infected with Lassa virus do not become ill, but they can shed the virus in their urine and faeces.
Because the clinical course of the disease is so variable, detection of the disease in affected patients has been difficult. When presence of the disease is confirmed in a community, however, prompt isolation of affected patients, good infection prevention and control practices, and rigorous contact tracing can stop outbreaks.
Lassa fever is known to be endemic in Benin (where it was diagnosed for the first time in November 2014), Ghana (diagnosed for the first time in October 2011), Guinea, Liberia, Mali (diagnosed for the first time in February 2009), Sierra Leone, and Nigeria, but probably exists in other West African countries as well.
The incubation period of Lassa fever ranges from 6–21 days. The onset of the disease, when it is symptomatic, is usually gradual, starting with fever, general weakness, and malaise. After a few days, headache, sore throat, muscle pain, chest pain, nausea, vomiting, diarrhoea, cough, and abdominal pain may follow. In severe cases facial swelling, fluid in the lung cavity, bleeding from the mouth, nose, vagina or gastrointestinal tract and low blood pressure may develop.
Protein may be noted in the urine. Shock, seizures, tremor, disorientation, and coma may be seen in the later stages. Deafness occurs in 25% of patients who survive the disease. In half of these cases, hearing returns partially after 1–3 months. Transient hair loss and gait disturbance may occur during recovery.
Death usually occurs within 14 days of onset in fatal cases. The disease is especially severe late in pregnancy, with maternal death and/or fetal loss occurring in more than 80% of cases during the third trimester.
Humans usually become infected with Lassa virus from exposure to urine or faeces of infected Mastomys rats. Lassa virus may also be spread between humans through direct contact with the blood, urine, faeces, or other bodily secretions of a person infected with Lassa fever. There is no epidemiological evidence supporting airborne spread between humans. Person-to-person transmission occurs in both community and health-care settings, where the virus may be spread by contaminated medical equipment, such as re-used needles. Sexual transmission of Lassa virus has been reported.
Lassa fever occurs in all age groups and both sexes. Persons at greatest risk are those living in rural areas where Mastomys are usually found, especially in communities with poor sanitation or crowded living conditions. Health workers are at risk if caring for Lassa fever patients in the absence of proper barrier nursing and infection prevention and control practices.
Because the symptoms of Lassa fever are so varied and non-specific, clinical diagnosis is often difficult, especially early in the course of the disease. Lassa fever is difficult to distinguish from other viral haemorrhagic fevers such as Ebola virus disease as well as other diseases that cause fever, including malaria, shigellosis, typhoid fever and yellow fever.
Definitive diagnosis requires testing that is available only in reference laboratories. Laboratory specimens may be hazardous and must be handled with extreme care. Lassa virus infections can only be diagnosed definitively in the laboratory using the following tests:
- reverse transcriptase polymerase chain reaction (RT-PCR) assay
- antibody enzyme-linked immunosorbent assay (ELISA)
- antigen detection tests
- virus isolation by cell culture.
The antiviral drug ribavirin seems to be an effective treatment for Lassa fever if given early on in the course of clinical illness. There is no evidence to support the role of ribavirin as post-exposure prophylactic treatment for Lassa fever.
There is currently no vaccine that protects against Lassa fever.
Prevention of Lassa fever relies on promoting good “community hygiene” to discourage rodents from entering homes. Effective measures include storing grain and other foodstuffs in rodent-proof containers, disposing of garbage far from the home, maintaining clean households and keeping cats. Because Mastomys are so abundant in endemic areas, it is not possible to completely eliminate them from the environment. Family members should always be careful to avoid contact with blood and body fluids while caring for sick persons.
In health-care settings, staff should always apply standard infection prevention and control precautions when caring for patients, regardless of their presumed diagnosis. These include basic hand hygiene, respiratory hygiene, use of personal protective equipment (to block splashes or other contact with infected materials), safe injection practices and safe burial practices.
Health-care workers caring for patients with suspected or confirmed Lassa fever should apply extra infection control measures to prevent contact with the patient’s blood and body fluids and contaminated surfaces or materials such as clothing and bedding. When in close contact (within 1 metre) of patients with Lassa fever, health-care workers should wear face protection (a face shield or a medical mask and goggles), a clean, non-sterile long-sleeved gown, and gloves (sterile gloves for some procedures).
Laboratory workers are also at risk. Samples taken from humans and animals for investigation of Lassa virus infection should be handled by trained staff and processed in suitably equipped laboratories under maximum biological containment conditions.
On rare occasions, travellers from areas where Lassa fever is endemic export the disease to other countries. Although malaria, typhoid fever, and many other tropical infections are much more common, the diagnosis of Lassa fever should be considered in febrile patients returning from West Africa, especially if they have had exposures in rural areas or hospitals in countries where Lassa fever is known to be endemic. Health-care workers seeing a patient suspected to have Lassa fever should immediately contact local and national experts for advice and to arrange for laboratory testing.
PHILIPPINES: Twelve residents who ate dog meat are under observation for rabies
Friday, April 6th, 2018
“….The residents….ate the dog meat which was cooked by the owner of the dog, who also slaughtered the animal himself…..
The owner reportedly did this after the dog bit him.
After 15 days, the owner passed away……”
Southern California was rattled Thursday by a magnitude 5.3 earthquake
Friday, April 6th, 2018Reemergence of Human Monkeypox in Nigeria, 2017
Friday, April 6th, 2018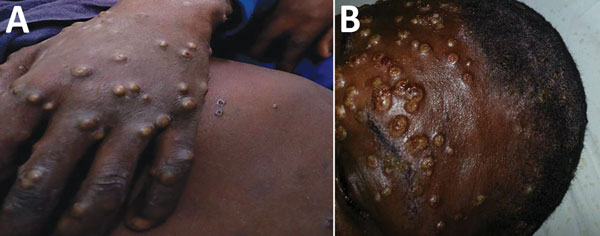
Yinka-Ogunleye A, Aruna O, Ogoina D, Aworabhi N, Eteng W, Badaru S, et al. Reemergence of human monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018 Jun [date cited]. https://doi.org/10.3201/eid2406.180017
Human monkeypox is a rare zoonotic infection caused by an orthopoxvirus and characterized by smallpox-like signs and symptoms (1). The disease is endemic to the Democratic Republic of the Congo. Reported outbreaks have occurred mainly in rural rainforest areas of the Congo basin and West Africa, caused by the Central and West African clades of the virus, respectively (1–6). The West African clade is associated with milder disease, fewer deaths, and limited human-to-human transmission. Since 1970, only ≈10 cases in West Africa had been reported; in 2003, a total of 81 cases (41% laboratory confirmed) were reported in the United States (2,7,8). In Nigeria, a case of human monkeypox in a 4-year-old child in the southeastern part of the country was reported in 1971 (4,5); no more cases in Nigeria had been reported since 1978 (2,6). We provide a preliminary report of a large outbreak of human monkeypox in Nigeria caused by the West African clade of monkeypox virus in 2017.
On September 22, 2017, the Nigeria Centre for Disease Control (NCDC) was notified of a suspected case of monkeypox; the patient had been admitted to the Niger Delta University Teaching Hospital, Bayelsa State, in the South South region of Nigeria. Outbreak investigations commenced immediately; isolation of the suspected case-patient, laboratory testing, and contact tracing were conducted.
The patient was an 11-year-old boy with an 11-day history of fever, generalized rash, headache, malaise, and sore throat. Physical examination revealed generalized well-circumscribed papulopustular rashes on the trunk, face, palms, and soles of the feet and subsequent umbilication, ulcerations, crusting, and scab formation. The patient had associated oral and nasal mucosal lesions and ulcers and accompanying generalized lymphadenopathy. Similar signs and symptoms, with varying degrees of severity, developed in 5 other family members living in the same household. The index case-patient and 2 of his siblings reported a history of having had contact with a neighbor’s monkey 1 month earlier, but it cannot be ascertained if the monkey was the source of their infection; the monkey had no known history of illness.
After identifying these cases as being suspected monkeypox, the NCDC immediately deployed epidemiologists to Bayelsa State to support detailed outbreak investigations. Health authorities in all states of the country were notified to establish enhanced surveillance based on a standardized case definition. As notification of suspected cases from other states increased, on October 9, 2017, the NCDC activated a national Emergency Operations Centre to coordinate the response to an unusual evolving outbreak. All relevant stakeholders (e.g., ministries of health, agriculture and animal health, and information) were mobilized for a robust response. The NCDC rapidly developed interim guidelines and protocols; disseminated them to all states; and implemented intensive surveillance, public sensitization, community mobilization, and case management accordingly across all states.
Laboratory diagnosis (by real-time PCR, IgM serology, and genomic sequencing) were initially undertaken at Institut Pasteur (Dakar, Senegal), Redeemer’s University Laboratory (Ede, Nigeria), and the US Centers for Disease Control and Prevention (Atlanta, GA, USA). Further diagnostics took place later at the NCDC National Reference Laboratory with technical support from the US Centers for Disease Control and Prevention.
On October 13, 2017, the NCDC received laboratory confirmation of a human monkeypox outbreak in Nigeria. As of November 17, 2017, a total of 146 suspected cases had been reported from 22 of the 36 states in Nigeria (Figure). Of the 134 samples (blood, lesion swab, and crust) collected during the reporting period, 107 samples were tested, and 42 samples from 14 states were laboratory confirmed as the West African clade of the monkeypox virus. Most (62%) of the laboratory-confirmed cases were in adults (21–40 years of age; median 30 years of age); the male:female ratio was 2:1. A 46-year-old male patient with confirmed monkeypox and a history of immunosuppressive illness died. For some patients with suspected (but ultimately deemed negative) cases of monkeypox, chickenpox (wild-type virus) was confirmed. Further analysis of the monkeypox-negative samples is ongoing.
Although detailed epidemiologic investigations to ascertain the source and route of transmission are ongoing, 3 family clusters were found, which might suggest some level of human-to-human transmission in this outbreak. For 1 of the families, the secondary attack rate was 71%. However, most patients had no obvious epidemiologic linkage or person-to-person contact, indicating a probable multiple-source outbreak or possibly previously unrecognized endemic disease. The zoonotic source(s) of the outbreak are currently unknown, and it is unclear what, if any, environmental or ecologic changes might have facilitated its sudden reemergence.
This large outbreak of West Africa clade human monkeypox (3,8,9) mostly affected adults. The NCDC continues response activities and investigations in collaboration with national and international partners. Further findings from our epidemiologic investigations and laboratory diagnostics, including genome sequencing, will add to the existing knowledge of West African monkeypox and help unravel uncertainties in the outbreak.