Archive for June, 2018
DRC: Ebola outbreak total grows to 53 cases at least
Tuesday, June 5th, 2018SITUATION ÉPIDÉMIOLOGIQUE Dimanche 03 juin 2018
La situation épidémiologique de la Maladie à Virus Ebola en date du 02 juin 2018 :
- Au total, 53 cas de fièvre hémorragique ont été signalés dans la région, dont 37 confirmés, 13 probables et 3 suspects.
- 1 nouveau cas suspect à Iboko
- 5 échantillons se sont révélés négatifs, dont 2 à Bikoro, 1 à Iboko et 2 à Wangata
- Aucun nouveau cas confirmé ce jour
- Aucun nouveau décès rapporté ce jour
Les analyses épidémiologiques ont permis d’identifier des contacts qui vivent dans les zones de santé voisines à Bikoro et Iboko. Ces contacts sont suivis et ont été conseillés de limiter leurs mouvements durant toute la période de suivi qui est de 21 jours.
Remarques
- Les tests négatifs sont systématiquement retirés du tableau récapitulatif.
- La catégorie des cas probables reprend tous les décès notifiés pour lesquels il n’a pas été possible d’obtenir des échantillons biologiques pour confirmation au laboratoire.
|
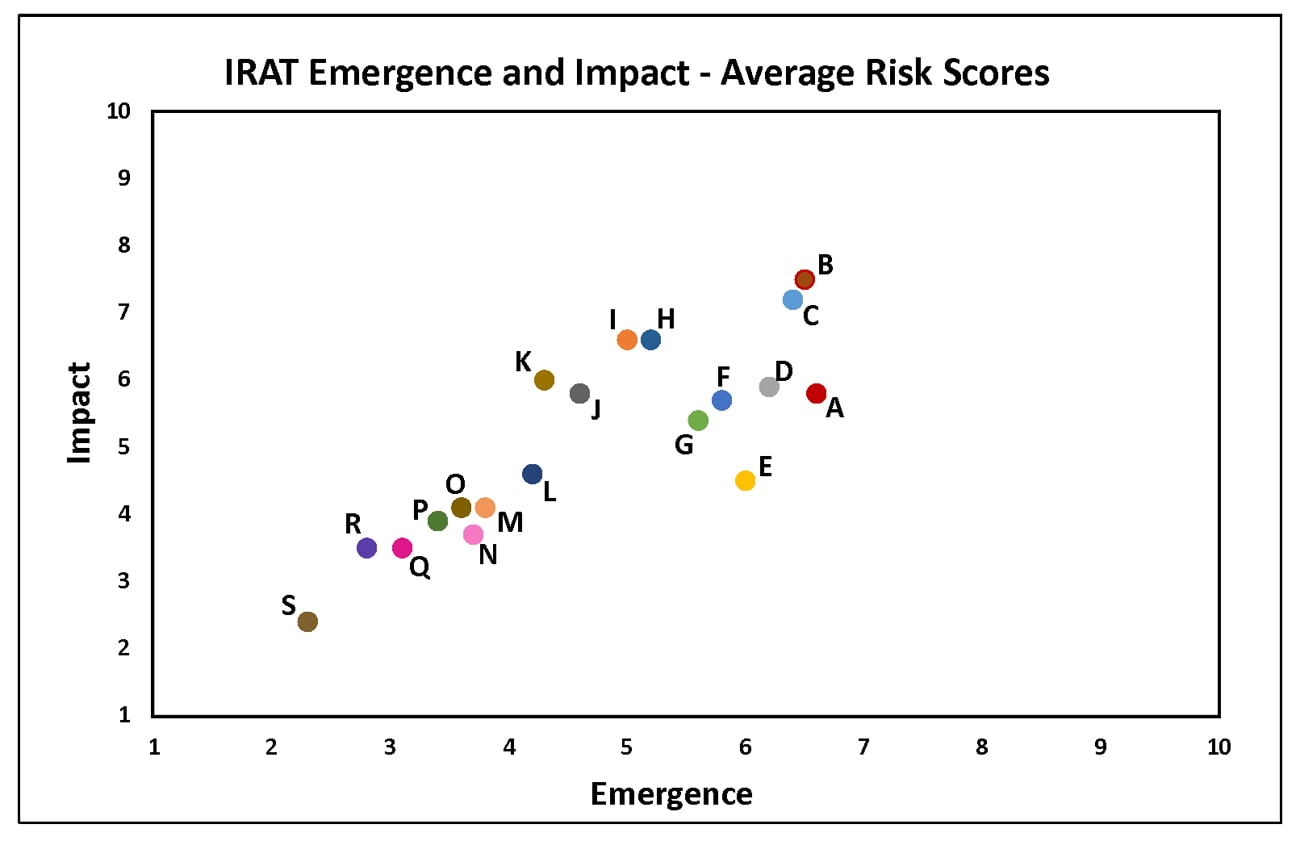
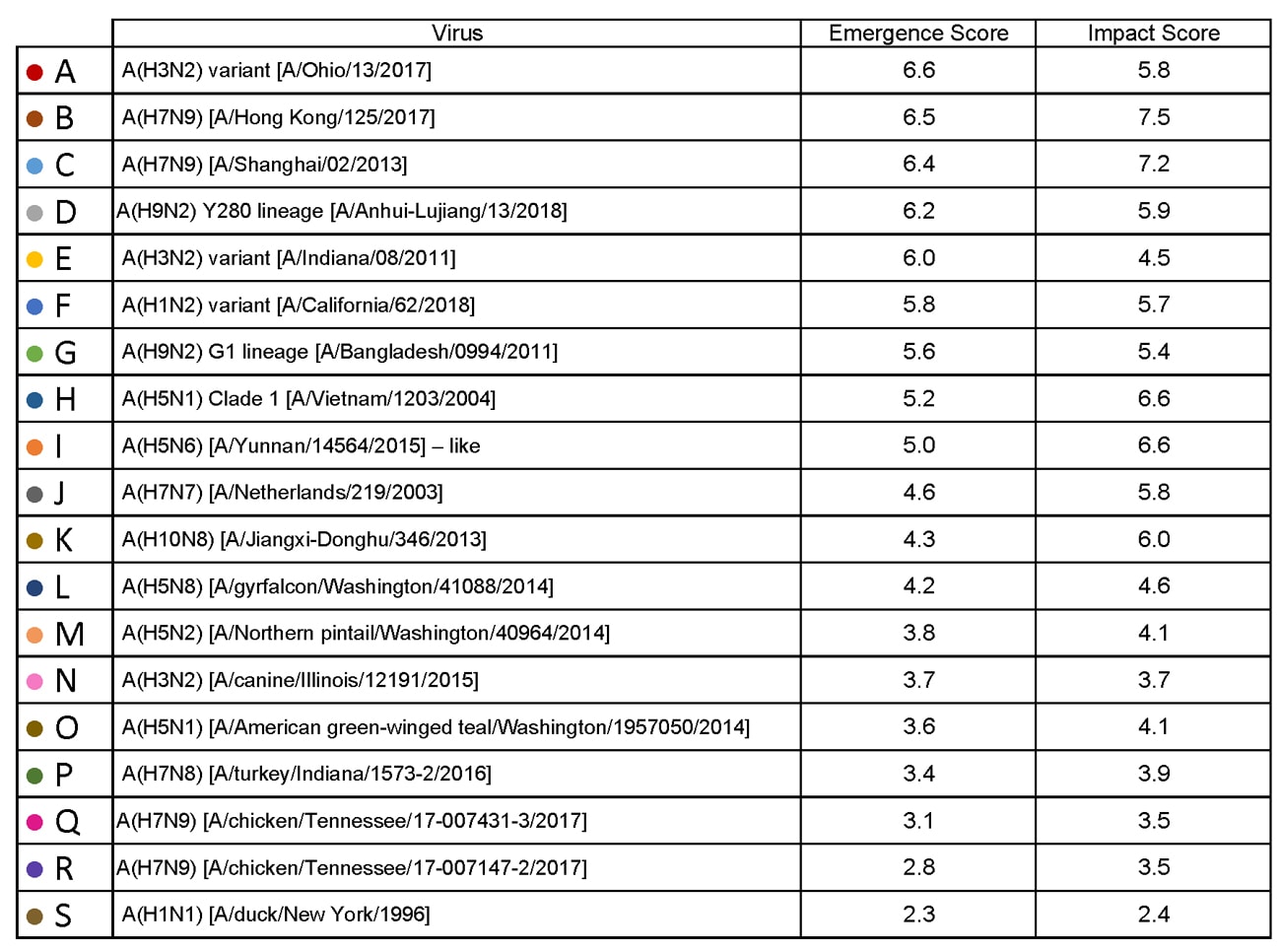
Summary of Influenza Risk Assessment Tool (IRAT) Results: 2018
Tuesday, June 5th, 2018The Influenza Risk Assessment Tool (IRAT) is an evaluation tool conceived by CDC and further developed with assistance from global animal and human health influenza experts. The IRAT is used to assess the potential pandemic risk posed by influenza A viruses that are not currently circulating in people. Input is provided by U.S. government animal and human health influenza experts. Information about the IRAT is available at Influenza Risk Assessment Tool (IRAT) Questions and Answers(https://www.cdc.gov/flu/pandemic-resources/national-strategy/risk-assessment.htm).
| Virus | Most Recent Date Evaluated | Potential Emergence Risk(https://www.cdc.gov/flu/pandemic-resources/tools/risk-assessment.htm#emergence-risk) | Potential Impact Risk(https://www.cdc.gov/flu/pandemic-resources/tools/risk-assessment.htm#impact-risk) | Overall Summary |
|---|---|---|---|---|
| H1N1 [A/duck/New York/1996] | Nov 2011 | 2.3 | 2.4 | Low |
| H3N2 variant [A/Indiana/08/2011] | Dec 2012 | 6.0 | 4.5 | Moderate |
| H3N2 [A/canine/Illinois/12191/2015] | June 2016 | 3.7 | 3.7 | Low |
| H5N1 Clade 1 [A/Vietnam/1203/2004] | Nov 2011 | 5.2 | 6.6 | Moderate |
| H5N1 [A/American green-winged teal/Washington/1957050/2014] | Mar 2015 | 3.6 | 4.1 | Low-Moderate |
| H5N2 [A/Northern pintail/Washington/40964/2014] | Mar 2015 | 3.8 | 4.1 | Low-Moderate |
| H5N6 [A/Yunnan/14564/2015] – like | Apr 2016 | 5.0 | 6.6 | Moderate |
| H5N8 [A/gyrfalcon/Washington/41088/2014] | Mar 2015 | 4.2 | 4.6 | Low-Moderate |
| H7N7 [A/Netherlands/219/2003] | Jun 2012 | 4.6 | 5.8 | Moderate |
| H7N8 [A/turkey/Indiana/1573-2/2016] | July 2017 | 3.4 | 3.9 | Low |
| H7N9 [A/chicken/Tennessee/17-007431-3/2017] | Oct 2017 | 3.1 | 3.5 | Low |
| H7N9 [A/ chicken/Tennessee /17-007147-2/2017] | Oct 2017 | 2.8 | 3.5 | Low |
| H7N9 [A/Hong Kong/125/2017] | May 2017 | 6.5 | 7.5 | Moderate-High |
| H7N9 [A/Shanghai/02/2013] | Apr 2016 | 6.4 | 7.2 | Moderate-High |
| H9N2 G1 lineage [A/Bangladesh/0994/2011] | Feb 2014 | 5.6 | 5.4 | Moderate |
| H10N8 [A/Jiangxi-Donghu/346/2013] | Feb 2014 | 4.3 | 6.0 | Moderate |
H1N1: [North American avian H1N1 [A/duck/New York/1996]
Avian influenza A viruses are designated as highly pathogenic avian influenza (HPAI) or low pathogenic avian influenza (LPAI) based on molecular characteristics of the virus and the ability of the virus to cause disease and death in chickens in a laboratory setting. North American avian H1N1 [A/duck/New York/1996] is a LPAI virus and in the context of the IRAT serves as an example of a low risk virus.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was in the low risk category (less than 3). Similarly the average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission also falls into the low risk range (less than 3).
H3N2 Variant:[A/Indiana/08/11]
Swine-origin flu viruses do not normally infect humans. However, sporadic human infections with swine-origin influenza viruses have occurred. When this happens, these viruses are called “variant viruses.” Influenza A H3N2 variant viruses (also known as “H3N2v” viruses) with the matrix (M) gene from the 2009 H1N1 pandemic virus were first detected in people in July 2011. The viruses were first identified in U.S. pigs in 2010. In 2011, 12 cases of H3N2v infection were detected in the United States. In 2012, 309 cases of H3N2v infection across 12 states were detected. The latest risk assessment for this virus was conducted in December 2012 and incorporated data regarding population immunity that was lacking a year earlier.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was in the moderate risk category (less than 6). The summary average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was in the low-moderate risk category (less than 5).
H3N2: [A/canine/Illinois/12191/2015]
The H3N2 canine influenza virus is an avian flu virus that adapted to infect dogs. This virus is different from human seasonal H3N2 viruses. Canine influenza A H3N2 virus was first detected in dogs in South Korea in 2007 and has since been reported in China and Thailand. It was first detected in dogs in the United States in April 2015(https://www.cdc.gov/flu/news/canine-influenza-update.htm). H3N2 canine influenza has reportedly infected some cats as well as dogs. There have been no reports of human cases.
Summary: The average summary risk score for the virus to achieve sustained human-to-human transmission was low risk (less than 4). The average summary risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was in the low risk range (less than 4). For a full report, click here[186 KB, 4 pages](https://www.cdc.gov/flu/pandemic-resources/pdf/cdc-irat-virus-canine-h3n2.pdf).
H5N1 clade 1: [A/Vietnam/1203/2004]
The first human cases of highly pathogenic avian influenza (HPAI) H5N1 virus were reported from Hong Kong in 1997. Since 2003, highly pathogenic H5N1 avian influenza viruses have caused over 850 laboratory-confirmed human cases; mortality among these cases was high. A risk assessment of this H5N1 clade 1 virus was conducted in 2011 soon after the IRAT was first developed and when 12 hemagglutinin (HA) clades were officially recognized.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was in the moderate risk category (less than 6). The summary average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was in the high-moderate risk category (less than 7).
H5N1: [A/American green winged teal/Washington/1957050/2014]
In December 2014, an H5N1 highly pathogenic avian influenza virus was first isolated from an American green-winged teal in the state of Washington. This virus is a recombinant virus containing four genes of Eurasian lineage (PB2, HA, NP and M) and four genes of North American lineage (PB1, PA, NA and NS).In February 2015, the Canadian government reported isolating this virus from a backyard flock in the Fraser Valley. When this risk assessment was conducted in 2015, these were the only reported isolations of this virus. There have been no reports of human cases.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was in the low risk category (less than 4). The summary average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was in the low-moderate risk category (less than 5).
H5N2: [A/Northern pintail/Washington/40964/2014]
In December 2014, an H5N2 highly pathogenic avian influenza virus was first reported by the Canadian government from commercial poultry in the Fraser Valley.Subsequently this virus was isolated from wild birds, captive wild birds, backyard flocks and commercial flocks in the United States.This virus is a recombinant virus composed of five Eurasian lineage (PB2, PA, HA, M and NS) genes and three North American lineage (PB1, NP and NA) genes. There have been no reports of human cases.
Summary: The average summary risk score for the virus to achieve sustained human-to-human transmission was low risk (less than 4).The average summary risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was in the low-moderate risk range (less than 5).
H5N6: [A/Yunnan/14564/2015 (H5N6-like)]
Between January 2014 and March 2016, there have been 10 human cases of H5N6 highly pathogenic avian influenza reported. Nine reportedly experienced severe disease and six died. Avian outbreaks of this virus were first reported from China in 2013. Subsequently avian outbreaks have been reported in at least three countries (China, Vietnam and Lao PDR) through 2015.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was in the moderate range (less than 6).The average summary risk score for the virus to significantly impact on public health if it were to achieve sustained human-to-human transmission fell in the moderate range (less than 7).
H5N8: [A/gyrfalcon/Washington/41088/2014]
In December 2014, an H5N8 highly pathogenic avian influenza virus was first isolated from a sample collected in the United States from a captive gyrfalcon.Subsequently this virus was isolated from wild birds, captive wild birds, backyard flocks and commercial flocks in the United States.This virus (clade 2.3.4.4) is similar to Eurasian lineage H5N8 viruses that have been isolated in South Korea, China, Japan, the Netherlands, the United Kingdom and Germany in late 2014-early 2015.There have been no reports of human cases.
Summary: The average risk score for the virus to achieve sustained human-to-human transmission was in the low-moderate range< (less than 5). The average summary risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission fell in the low-moderate range (less than 5).
In 2003 the Netherlands reported highly pathogenic avian influenza (HPAI) in approximately 255 commercial flocks.Coinciding with human activities around these infected flocks, 89 human cases of H7N7 were identified.Cases primarily reported conjunctivitis, although a few also reported mild influenza-like illness.There was one death.
Summary: The summary average risk score for this virus to achieve sustained human-to-human transmission was in the low-moderate risk range (less than 5). The summary average risk score for this virus to significantly impact the public’s health if it were to achieve sustained human-to-human transmission fell in the moderate risk range (less than 7).
H7N8: [A/turkey/Indiana/1573-2/2016]
In January 2016, a highly pathogenic avian influenza (HPAI) virus of North American lineage was identified in a turkey flock in Indiana. Putative low pathogenic avian influenza (LPAI) viruses similar to A/turkey/Indiana/1573-2/2016 were subsequently isolated from 9 other turkey flocks in the area. There were no reports of human cases associated with this virus at the time of the IRAT scoring.
Summary: A risk assessment of this LPAI virus was conducted in July 2017. The overall IRAT risk assessment score for this virus falls into the low risk category (< 4). The summary average risk score for the virus to achieve sustained human-to-human transmission is in the low risk category (3.4). The summary average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was also in the low risk category (3.9).
Low pathogenic avian influenza (LPAI) H7N9 viruses were first reported from China in March 2013. These viruses were first scored using the IRAT in April 2013, and then annually in 2014, 2015, and 2016 with no change in overall risk scores. Between October 2016 and May 2017 evidence of two divergent lineages of these viruses was detected – the Pearl River Delta lineage and the Yangtze River Delta lineage. The IRAT was used to assess LPAI H7N9 [A/Hong Kong/125/2017], a representative of the Yangtze River Delta viruses.
Summary: A risk assessment of H7N9 [A/Hong Kong/125/2017] was conducted in May 2017. The overall IRAT risk assessment score for this virus falls into the moderate-high risk category and is similar to the scores for the previous H7N9 viruses. The summary average risk score for the virus to achieve sustained human-to-human transmission is in the moderate risk category (less than 7). The summary average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was in the moderate-high risk category (less than 8).
H7N9: Avian H7N9 [A/Shanghai/02/2013]
On 31 March 2013, the China Health and Family Planning Commission notified the World Health Organization (WHO) of three cases of human infection with influenza H7N9. As of August 2016, the WHO has received reports of 821 cases, 305 have died. This low pathogenic avian influenza virus was rescored most recently in April 2016 with no substantive change in risk scores since May 2013.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was in the moderate risk category (less than 7). The average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission fell in the high-moderate risk range (less than 8).
H7N9: Low Pathogenic North American avian [A/chicken/Tennessee/17-007431-3/2017]
Surveillance conducted in March 2017 during the investigation of a highly pathogenic avian influenza (HPAI) A(H7N9) virus in commercial poultry in Tennessee revealed the contemporaneous presence of North American lineage low pathogenic avian influenza (LPAI) A(H7N9) virus in commercial and backyard poultry flocks in Tennessee and three other states. The outbreak in poultry appeared limited with no further detections in subsequent surveillance. There were no reports of human cases associated with this virus.
Summary: A risk assessment this North American lineage LPAI A(H7N9) virus was conducted in October 2017. The overall IRAT risk assessment score for this virus falls into the low risk category (< 4). The summary average risk score for the virus to achieve sustained human-to-human transmission was in the low risk category (score 3.1). The average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was between the low to low-moderate range (score 3.5). For a full report, click here[228 KB, 4 pages](https://www.cdc.gov/flu/pandemic-resources/pdf/cdc-irat-virus-lpai.pdf).
H7N9: High Pathogenic North American avian [A/chicken/Tennessee/17-007147-2/2017]
In March 2017, the U.S. Department of Agriculture (USDA) reported the detection of a highly pathogenic avian influenza (HPAI) A(H7N9) virus in 2 commercial poultry flocks in Tennessee. Full genome sequence analysis indicated that all eight gene segments of the virus were of North American wild bird lineage and genetically distinct from the lineage of influenza A(H7N9) viruses infecting poultry and humans in China since 2013. The outbreak investigation revealed that a related North American low pathogenic avian influenza A(H7N9) was circulating in poultry prior to the detection of the HPAI A(H7N9). There were no reports of human cases associated with this virus.
Summary: A risk assessment this North American lineage HPAI A(H7N9) virus was conducted in October 2017. The overall IRAT risk assessment score for this virus falls into the low risk category (< 4). The summary average risk score for the virus to achieve sustained human-to-human transmission is in the low risk category (2.8). The summary average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was also in the low risk category (3.5). For a full report, click here[225 KB, 4 pages](https://www.cdc.gov/flu/pandemic-resources/pdf/cdc-irat-virus-hpai.pdf).
H9N2: Avian H9N2 G1 lineage [A/Bangladesh/0994/2011]
Human infections with influenza AH9N2 virus have been reported sporadically, cases reportedly exhibited mild influenza-like illness. Historically these low pathogenic avian influenza viruses have been isolated from wild and domestic birds. In response to these reports, a risk assessment of this H9N2 influenza virus was conducted in 2014.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was in the moderate risk category (less than 6). The summary average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission also fell in the moderate risk range (less than 6).
H10N8: Avian H10N8 [A/Jiangxi-Donghu/346/2013]
Two human infections with influenza A(H10N8) virus were reported by the China Health and Family Planning Commission in 2013 and 2014 (one each year). Both cases were hospitalized and one died. Historically low pathogenic avian influenza H10 and N8 viruses have been recovered from birds. A risk assessment of the H10N8 influenza was conducted in 2014.
Summary: The summary average risk score for the virus to achieve sustained human-to-human transmission was low-moderate (less than 5). The average risk score for the virus to significantly impact public health if it were to achieve sustained human-to-human transmission was in the moderate risk range (less than 7).
At least six people are dead and 20 were injured after Volcan de Fuego erupts
Monday, June 4th, 2018https://www.youtube.com/watch?v=ZOBK0e1iiKE
https://www.youtube.com/watch?v=_PKEB-a_Mxg
Volcán de Fuego y sus erupciones… pic.twitter.com/6T9TxQ6hOo
— Guía espiritual (Demiurgo). (@RB_Sativa) June 3, 2018
Climate change and dengue fever in Latin America
Monday, June 4th, 2018Felipe J. Colón-González el al., “Limiting global-mean temperature increase to 1.5–2 °C could reduce the incidence and spatial spread of dengue fever in Latin America,” PNAS (2018).
Read more at: https://phys.org/news/2018-05-limiting-global-millions-dengue-fever.html#jCp
“……We show that policies to limit global warming to 2 °C could reduce dengue cases by about 2.8 (0.8–7.4) million cases per year by the end of the century compared with a no-policy scenario that warms by 3.7 °C. Limiting warming further to 1.5 °C produces an additional drop in cases of about 0.5 (0.2–1.1) million per year…..”
Egypt is wiping out Hepatitis C at an unprecedented pace. How?
Monday, June 4th, 2018“…..The hepatitis C epidemic in Egypt—the country with the highest prevalence of the disease in the world—started around 50 years ago, when the government was attempting to get rid of one plague and ended up substituting it for another. For millennia the Nile Delta has been an ideal breeding ground for schistosomiasis, a parasite spread to humans by freshwater snails. In the mid-20th century, the Egyptian government conducted multiple mass-treatment campaigns using an injectable emetic—and needles were repeatedly reused. Hepatitis C virus, not yet known but transmitted efficiently by blood, was inadvertently spread to many citizens. By 2008, one in 10 Egyptians had chronic hepatitis C…..By 2015 hepatitis C accounted for 40,000 deaths per year in Egypt—7.6 percent of all deaths there—and depressed national GDP growth by 1.5 percent……..”

All about tornadoes in the US
Sunday, June 3rd, 2018India: One more person confirmed to have contracted the Nipah Virus infection passed away in Kerala, taking the death toll to 17.
Sunday, June 3rd, 2018“……Suspecting a second wave of the infection, the authorities have scaled up the number of people, who are under observation for Nipah Virus to 1407.
Meanwhile, the medicines from Australia to fight the Nipah Virus – M 102.4 human monoclonal antibody arrived in Kozhikode on Thursday night and will be administered to patients as per the treatment protocol. ……”
Nipah virus update – India
Sunday, June 3rd, 2018On 19 May 2018, three deaths due to Nipah virus infection were reported in Kozhikode District, Kerala State, India. The three deaths occurred in a family cluster and a fourth death was subsequently reported in a health care worker who was involved in treatment of the family in the local hospital. Laboratory testing of throat swabs, urine and blood samples collected from four suspected patients has been conducted by the National Institute of Virology in Pune; three of the four reported deaths were confirmed positive for Nipah virus (NiV) by real-time polymerase chain reaction (RT-PCR) and IgM Elisa for NiV.
The field investigation team found bats living in the abandoned water well on the premises of a new house where the family had plans to move after renovation. One bat was caught and sent to the National Institute of Virology, Pune for laboratory testing.
As of 28 May 2018 and since the beginning of the outbreak, as a result of further investigations and contact tracing, 15 people have tested positive for NiV in Kozhikode and Malappuram districts, Kerala State. Of the 15 laboratory-confirmed cases, two are hospitalized and thirteen have died, including the health care worker who was involved in treatment of the deceased. As of 28 May, 13 deaths have been reported: three from Malappuram District and ten from Kozhikode District. One deceased case, the index case, could not be tested but was epidemiologically linked to a confirmed case. There are 16 suspected cases identified through contact tracing who are under observation while their laboratory results are pending and at least 753 additional people, including health care workers, under observation. Laboratory testing is being conducted by the Manipal Institute of Virus Research and the National Institute of Virology, Pune; both laboratories have advanced capacity for RT-PCR.
In the current outbreak, acute respiratory distress syndrome and encephalitis have been observed.
This is the first NiV outbreak reported in Kerala State and third NiV outbreak known to have occurred in India, with the most recent outbreak reported in 2007.
Public health response
Government response
- A multi-disciplinary central team from the National Centre for Disease Control was sent to Kerala to investigate and respond. Contact tracing has been initiated. Infection prevention and control measures have been strengthened in health facilities.
- Acute fever and acute encephalitis syndrome (AES) surveillance have been enhanced across the state. Hospital and community surveillance have also been strengthened in Kerala.
- The Virus Research Diagnostic Laboratory at Manipal Hospital and the National Institute of Virology are conducting laboratory testing to confirm cases.
- The government is coordinating with all relevant sectors including zoonosis, wildlife, animal husbandry, human health, clinicians, pulmonologists, neurologists and private sector.
- Risk communication messages are being delivered to the community, public, stakeholders, and partners. The Ministry of Health and Family Welfare (MoHFW) has shared guidelines drafted by the National Centre for Disease Control with states and relevant stakeholders and also posted them on the MoHFW website.
WHO response
- WHO is in contact with national authorities and continues to closely monitor this event.
- At the request of the MoHFW, WHO has shared materials, especially risk communication materials on Nipah virus, including those used in Bangladesh.
- The MoHFW is conducting preliminary investigations and may request that WHO support the response.
- The MoHFW is coordinating a multi-dimensional investigation and may request support from WHO.
WHO risk assessment
NiV infection is an emerging zoonotic disease of public health importance in the WHO South-East Asia Region with a high case fatality rate estimated to be between 40 and 75%; however, this rate can vary by outbreak depending on local capabilities for epidemiological surveillance and clinical management. NiV was first recognized in 1998-1999 during an outbreak among pig farmers in Malaysia and Singapore. No subsequent outbreaks have been reported in Malaysia or Singapore since 1999. NiV was first recognized in India and Bangladesh in 2001; since then, nearly annual outbreaks have occurred in Bangladesh. The disease has been identified periodically in eastern India (2001, 2007).
Limited human-to-human transmission of NiV can occur among family members and health workers who treat infected patients. Large fruit bats of the genus Pteropus are the natural reservoirs of NiV and given the wide distribution of the species and migration of the locally-abundant fruit bats in India, the risk of exposure to NiV is high. Nevertheless, previous outbreaks in affected countries have had a strong seasonal pattern and a limited geographical range.
Possible routes of transmission for this outbreak include consumption of fruits partially eaten by the bats, exposure to the virus by bats or human-to-human transmission through unprotected close contact in the community or hospital. Many cases identified in the current outbreak were infected through direct unprotected contact with other infected persons.
Given that India has faced and contained Nipah virus outbreaks before, the country has the capacity to rapidly respond and verify cases with laboratory testing. At the moment, the outbreak is localized and WHO assesses the risk to be low at the national and regional levels.
WHO advice
Currently, there are no specific treatments available for Nipah virus disease and care is supportive. Intensive supportive care is recommended to treat severe respiratory and neurologic complications.
NiV infection can be prevented by avoiding exposure to sick pigs and bats in endemic areas, and by avoiding consuming fruits partially-eaten by infected bats or drinking raw date palm sap/toddy/juice.
In health care settings, staff should consistently implement standard infection prevention and control measures when caring for patients to prevent nosocomial infections. Health care workers caring for a patient suspected to have NiV fever should immediately contact local and national experts for guidance and to arrange for laboratory testing.
Research is needed to better understand the ecology of bats and NiV.
WHO advises against the application of any travel or trade restrictions on India based on the information currently available on this event.
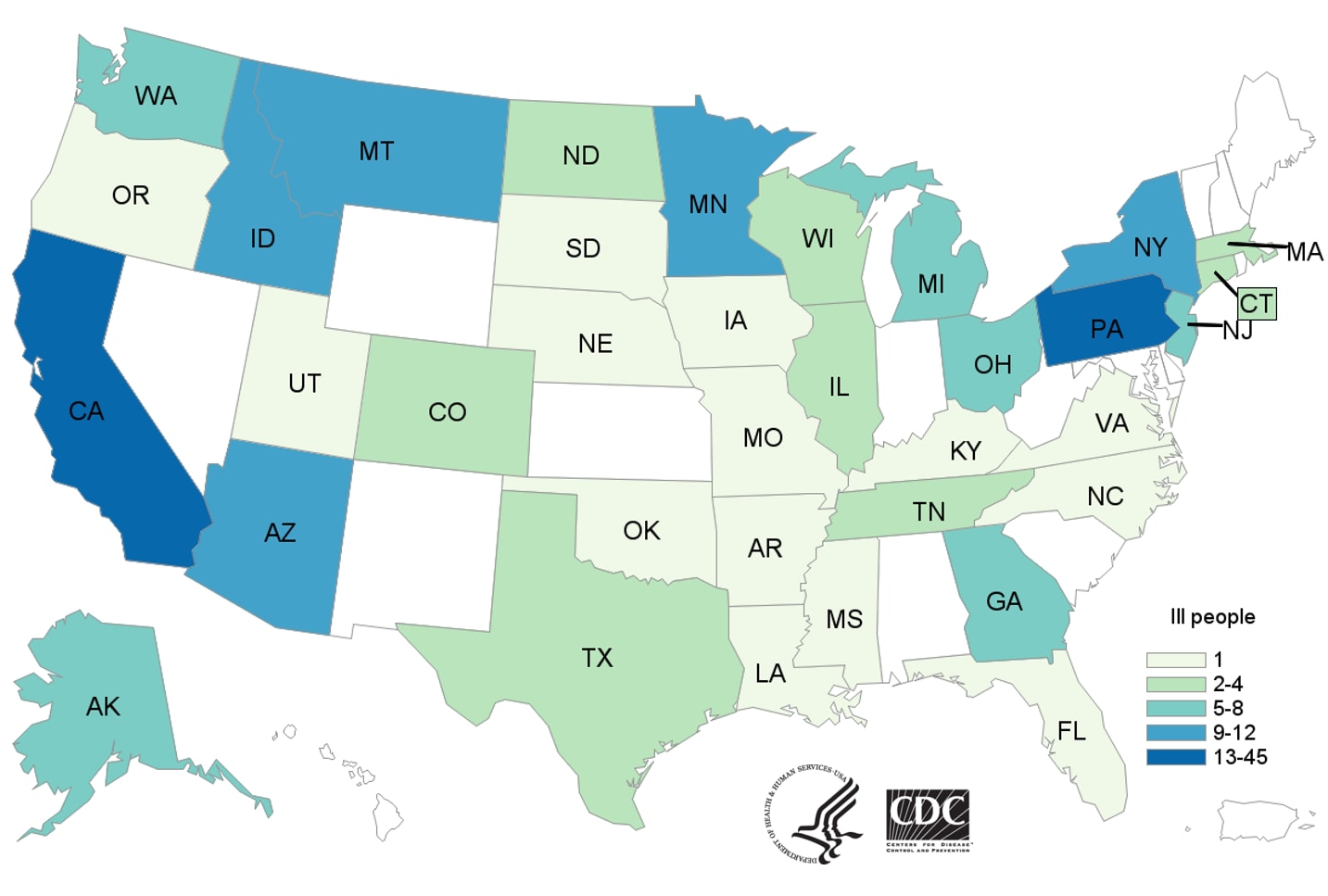
E. coli O157:H7 Infections Linked to Romaine Lettuce
Saturday, June 2nd, 2018What’s New?
- Twenty-five more ill people from 13 states were added to this investigation since the last update on May 16, 2018.
- Three more states have reported ill people: Arkansas, North Carolina, and Oklahoma.
- Four more deaths were reported from Arkansas (1), Minnesota (2), and New York (1).