Archive for March, 2019
Influenza anti-virals
Sunday, March 31st, 2019CDC recommends antiviral medications for treatment of influenza, regardless of a patient’s influenza vaccination status. Antiviral treatment has been shown to have clinical and public health benefit in reducing illness and severe outcomes of influenza based on evidence from randomized controlled trials, meta-analyses of randomized controlled trials, and observational studies during past influenza seasons and during the 2009 H1N1 pandemic [2–9]. Influenza antiviral medications are most effective in treating influenza and reducing complications when treatment is started early (within 48 hours of illness onset). However, some studies suggest clinical benefit among hospitalized patients and young children with febrile illness even when treatment starts three to five days after illness onset [10–16].
Recommendations
- All Hospitalized, Severely Ill, and High-Risk Patients with Suspected or Confirmed Influenza Should Be Treated with Antivirals
Antiviral treatment is recommended as early as possible for any patient with suspected or confirmed influenza who:1) Is hospitalized—treatment is recommended for all hospitalized patients;2) Has severe, complicated, or progressive illness—this may include outpatients with severe or prolonged progressive symptoms or patients who develop complications such as pneumonia but who are not hospitalized;3) Is at high risk for influenza complications but not hospitalized—this includes- Adults 65 years and older.
- Children younger than two years. Although all children younger than five years are considered at higher risk for complications from influenza, the highest risk is for those younger than two years.
- People with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematological (including sickle cell disease), and metabolic disorders (including diabetes mellitus).
- People with neurologic and neurodevelopment conditions (including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy [seizure disorders], stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord injury).
- People with immunosuppression, including that caused by medications or by HIV infection.
- Women who are pregnant or postpartum (within two weeks after delivery).
- People younger than 19 years who are receiving long-term aspirin therapy.
- American Indians and Alaska Natives.
- People with extreme obesity (i.e., body-mass index is equal to or greater than 40).
- Residents of nursing homes and other chronic-care facilities.
- Antivirals in Non-High Risk Patients with Uncomplicated Influenza
Antiviral treatment can benefit other individuals with influenza. While current guidance focuses on antiviral treatment of those with severe illness or at high risk of complications, antiviral treatment may be prescribed for any previously healthy (non-high risk) outpatient with suspected or confirmed influenza who presents within two days after illness onset. Clinical judgment—considering the patient’s disease severity and progression, age, likelihood of influenza, and time since onset of symptoms—is important when making antiviral treatment decisions for outpatients who are not at increased risk for influenza complications. - Choice of Antiviral Medication
Four influenza antiviral medications approved by the U.S. Food and Drug Administration (FDA) are recommended for use in the United States during the 2018-2019 influenza season. Three drugs are chemically related antiviral medications known as neuraminidase inhibitors that block the viral neuraminidase enzyme and have activity against both influenza A and B viruses: oral oseltamivir phosphate (available as a generic version or under the trade name Tamiflu®), inhaled zanamivir (trade name Relenza®), and intravenous peramivir (trade name Rapivab®). The fourth drug is oral baloxavir marboxil (trade name Xofluza®), which is active against both influenza A and B viruses but has a different mechanism of action. Baloxavir is a cap-dependent endonuclease inhibitor that interferes with viral RNA transcription and blocks virus replication. Recommended ages for treatment and prevention with antiviral medications are summarized in the table below. Dosing and more detailed treatment considerations can be found in the Summary of Influenza Antiviral Treatment Recommendations for Clinicians (http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm). - Antiviral Route Treatment Chemoprophylaxis Most Common Adverse Events (Recommended Age)
- Oseltamivir oral and enteric any age >3 months nausea, vomiting, headache*
- Zanamivir inhaled >7 years >5 years bronchospasm
- Peramivir intravenous >2 years n/a diarrhea
- Baloxavir oral >12 years n/a none more common than placebo *Nausea and vomiting are generally transient and can be mitigated if oseltamivir is taken with food n/a = not applicable
- For outpatients with acute uncomplicated influenza, oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir may be used for treatment.
The recommended treatment course for uncomplicated influenza is- Two doses per day of oral oseltamivir for five days, or
- Two doses per day of inhaled zanamivir for five days, or
- One dose per day of intravenous peramivir for one day, or
- One dose per day of oral baloxavir for one day.
- Oral or enterically-administered oseltamivir is the only recommended antiviral medication for treatment of hospitalized patients with suspected or confirmed influenza and patients with severe or complicated illness with suspected or confirmed influenza (e.g., pneumonia, exacerbation of underlying chronic medical condition) who are not hospitalized. There are insufficient data for inhaled zanamivir, intravenous peramivir, and oral baloxavir in patients with severe influenza disease.
- Oral oseltamivir is preferred for treatment of pregnant women. Pregnant women are recommended to receive the same antiviral dosing as non-pregnant people. Baloxavir is not recommended for the treatment of pregnant women or breastfeeding mothers, as there are no available efficacy or safety data.
- Timing of Treatment and Implications for Patient Evaluation, Treatment, and Testing
Clinical benefit is greatest when antiviral treatment is administered as early as possible after illness onset. Therefore, antiviral treatment should be started as soon as possible after illness onset and should not be delayed, even for a few hours to wait for the results of testing. Ideally, treatment should be initiated within 48 hours of symptom onset. However, antiviral treatment initiated later than 48 hours after illness onset can still be beneficial for some patients. Because of the importance of early treatment, decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza. Therefore, empiric antiviral treatment should be initiated as soon as possible when there is known influenza activity in the community. A history of current season influenza vaccination does not exclude a diagnosis of influenza in an ill child or adult. High-risk patients should be advised to call their provider promptly if they have symptoms of influenza.
References
- Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–951. https://www.sciencedirect.com/science/article/pii/S1473309916001298?via%3DihubExternal
- Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl J Med 2018; 379:913–923. https://www.nejm.org/doi/full/10.1056/NEJMoa1716197External
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis 2018; 68:e1–e47. https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/ciy866External
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. The Lancet 2015; 385:1729–1737. https://www.sciencedirect.com/science/article/pii/S0140673614624491?via%3DihubExternal
- Malosh RE, Martin ET, Heikkinen T, Monto AS, Brooks WA, Whitley RJ. Efficacy and Safety of Oseltamivir in Children: Systematic Review and Individual Patient Data Meta-analysis of Randomized Controlled Trials. Clin Infect Dis 2018; 66:1492–1500. https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/cix1040External
- Hsu J, Santesso N, Brozek J, et al. Antivirals for influenza: a summary of a systematic review and meta-analysis of observational studies. Influenza Other Respir Viruses 2013; 7 Suppl 2:76–81. https://onlinelibrary.wiley.com/doi/full/10.1111/irv.12085External
- Doll MK, Winters N, Kraicer-Melamed H, Boikos C, Quach C, Gore G. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother 2017; 72:2990–3007. https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkx271External
- Venkatesan S, Myles PR, Leonardi-Bee J, et al. Impact of Outpatient Neuraminidase Inhibitor Treatment in Patients Infected With Influenza A(H1N1)pdm09 at High Risk of Hospitalization: An Individual Participant Data Metaanalysis. Clin Infect Dis 2017; 64:1328–1334. https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/cix127External
- Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2:395–404. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(14)70041-4/fulltextExternal
- Lee N, Choi KW, Chan PKS, et al. Outcomes of adults hospitalised with severe influenza. Thorax 2010; 65:510–515. https://thorax.bmj.com/content/65/6/510External
- Lee N, Cockram CS, Chan PKS, Hui DSC, Sung JJY, Choi KW. Antiviral Treatment for Patients Hospitalized with Severe Influenza Infection May Affect Clinical Outcomes. Clin Infect Dis 2008; 46:1323–1324. https://academic.oup.com/cid/article/46/8/1323/364997External
- Lee EH, Wu C, Lee EU, et al. Fatalities Associated with the 2009 H1N1 Influenza A Virus in New York City. Clin Infect Dis 2010; 50:1498–1504. https://academic.oup.com/cid/article/50/11/1498/507049External
- Louie JK, Yang S, Acosta M, et al. Treatment With Neuraminidase Inhibitors for Critically Ill Patients With Influenza A (H1N1)pdm09. Clin Infect Dis 2012; 55:1198–1204. https://academic.oup.com/cid/article/55/9/1198/435273External
- McGeer A, Green KA, Plevneshi A, et al. Antiviral Therapy and Outcomes of Influenza Requiring Hospitalization in Ontario, Canada. Clin Infect Dis 2007; 45:1568–1575. https://academic.oup.com/cid/article/45/12/1568/303324External
- Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 Influenza A(H1N1) Virus Illness Among Pregnant Women in the United States. JAMA 2010; 303:1517–1525. https://jamanetwork.com/journals/jama/fullarticle/185713External
- Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis 2014; 14:109–118. https://www.ncbi.nlm.nih.gov/pubmed/2426859External
Antiviral agents
Sunday, March 31st, 2019| Antiviral | Route | Treatment | Chemoprophylaxis | Most Common Adverse Events |
| (Recommended Age) | ||||
| Oseltamivir | oral and enteric | any age | >3 months | nausea, vomiting, headache* |
| Zanamivir | inhaled | >7 years | >5 years | bronchospasm |
| Peramivir | intravenous | >2 years | n/a | diarrhea |
| Baloxavir | oral | >12 years | n/a | none more common than placebo |
| *Nausea and vomiting are generally transient and can be mitigated if oseltamivir is taken with food n/a = not applicable |
A Case of Neurocysticercosis
Saturday, March 30th, 2019Disseminated Cysticercosis
- Nishanth Dev, M.D.,
- and S. Zafar Abbas, M.D.
March 28, 2019
N Engl J Med 2019; 380:1267
DOI: 10.1056/NEJMicm1810953
An 18-year-old man presented…..generalized tonic–clonic seizures. His parents reported that he had been having pain in the right groin for 1 week. On physical examination, the patient was confused. He had swelling over the right eye and tenderness in the right testis. Magnetic resonance imaging of the head showed numerous well-defined cystic lesions throughout the cerebral cortex and the brain stem and cerebellum that were consistent with neurocysticercosis……. Despite treatment with dexamethasone and antiepileptic medications, the patient died 2 weeks later.
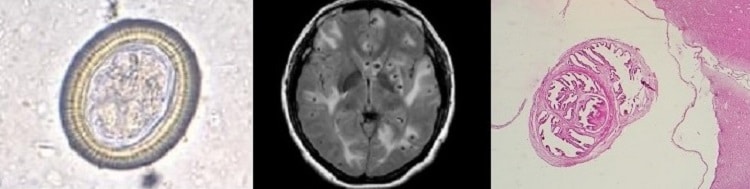
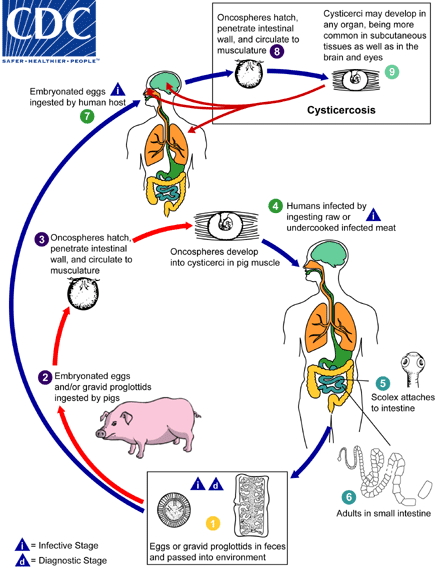
Cysticercosis is an infection of both humans and pigs with the larval stages of the parasitic cestode, Taenia solium. This infection is caused by ingestion of eggs shed in the feces of a human tapeworm carrier
. Pigs and humans become infected by ingesting eggs or gravid proglottids
,
. Humans are infected either by ingestion of food contaminated with feces, or by autoinfection. In the latter case, a human infected with adult T. solium can ingest eggs produced by that tapeworm, either through fecal contamination or, possibly, from proglottids carried into the stomach by reverse peristalsis. Once eggs are ingested, oncospheres hatch in the intestine
,
invade the intestinal wall, and migrate to striated muscles, as well as the brain, liver, and other tissues, where they develop into cysticerci
. In humans, cysts can cause serious sequellae if they localize in the brain, resulting in neurocysticercosis. The parasite life cycle is completed, resulting in human tapeworm infection, when humans ingest undercooked pork containing cysticerci
. Cysts evaginate and attach to the small intestine by their scolex
. Adult tapeworms develop, (up to 2 to 7 m in length and produce less than 1000 proglottids, each with approximately 50,000 eggs) and reside in the small intestine for years
.
Saudi Arabia: From 1 through 28 February 2019, 68 additional cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection, including 10 deaths.
Saturday, March 30th, 2019This Disease Outbreak News update describes the 19 cases. Among these cases, fifteen were sporadic, and four were reported as part of two unrelated clusters. Cluster 1 involved two cases in Buridah city; and Cluster 2 involved two cases in Riyadh city. The link below provides details of the 19 reported cases.
A separate Disease Outbreak News will provide an update on the outbreak in Wadi Aldwasir which affected 49 cases and resulted in seven deaths in February making a total of 52 cases since the onset of the outbreak.
From 2012 through 28 February 2019, the total number of laboratory-confirmed MERS cases reported globally to WHO is 2374 with 823 associated deaths. The global number reflects the total number of laboratory-confirmed cases reported to WHO under IHR to date. The total number of deaths includes the deaths that WHO is aware of to date through follow-up with affected member states.
WHO risk assessment
Infection with MERS-CoV can cause severe disease resulting in high morbidity and mortality. Humans are infected with MERS-CoV from direct or indirect contact with infected dromedary camels. MERS-CoV has demonstrated the ability to transmit between humans, especially from close unprotected contact with infected patients. So far, the observed non-sustained human-to-human transmission has occurred mainly in health care settings.
The notification of these additional cases does not change WHO’s overall risk assessment of MERS. WHO expects that additional cases of MERS will be reported from the Middle East, and that cases will continue to be exported to other countries by individuals who might acquire the infection after exposure to dromedary camels, animal products (e.g. consumption of camel’s raw milk), or humans (e.g. in a health care setting). WHO continues to monitor the epidemiological situation and conducts risk assessment based on the latest available information.
WHO advice
Based on the current situation and available information, WHO encourages all Member States to continue their surveillance for acute respiratory infections and to carefully review any unusual patterns.
Infection prevention and control (IPC) measures are critical to prevent the possible spread of MERS-CoV in health care facilities. It is not always possible to identify patients with MERS-CoV infection early because like other respiratory infections, the early symptoms of MERS are non-specific. Therefore, healthcare workers should always apply standard precautions consistently with all patients, regardless of their diagnosis. Droplet precautions should be added to the standard precautions when providing care to patients with symptoms of acute respiratory infection; contact precautions and eye protection should be added when caring for probable or confirmed cases of MERS; airborne precautions should be applied when performing aerosol generating procedures.
Early identification, case management and isolation, together with appropriate infection prevention and control measures can prevent human-to-human transmission of MERS-CoV.
WHO recommends that comprehensive identification, follow up and testing of all contacts of MERS patients be conducted, if feasible, regardless of the development of symptoms since approximately 20% of all reported MERS cases have been reported as mild or asymptomatic. The role of asymptomatic MERS-CoV infection in transmission is not well understood. However, reports of transmission from an asymptomatic MERS patient to another individual have been documented.
MERS causes more severe disease in people with underlying chronic medical conditions such as diabetes mellitus, renal failure, chronic lung disease, and compromised immune systems. Therefore, people with these underlying medical conditions should avoid close unprotected contact with animals, particularly dromedary camels, when visiting farms, markets, or barn areas where the virus is known to be potentially circulating. General hygiene measures, such as regular hand washing before and after touching animals and avoiding contact with sick animals, should be adhered to.
Food hygiene practices should be observed. People should avoid drinking camel’s raw milk or camel urine or eating camel meat that has not been properly cooked.
WHO does not advise special screening at points of entry with regard to this event nor does it currently recommend the application of any travel or trade restrictions.
The World Health Organization (WHO) Western Pacific Region: Will the measles epidemic grow out of control?
Saturday, March 30th, 2019Measles outbreaks in the World Health Organization (WHO) Western Pacific Region are putting babies, children and young people at risk and threatening progress towards wiping out the disease.
The Region had historically low levels of measles cases and no major outbreaks in 2017. This landmark decline was achieved through steady efforts to vaccinate all children against measles, but last year, measles cases in the Region increased by 250%, and more than two-thirds of cases were in the Philippines. So far this year, the Philippines has reported 23 000 cases with 333 deaths — already more than all of last year. Tragically, most of the cases were among children under 5 years old.
Measles can cause debilitating complications, including encephalitis, severe diarrhoea and dehydration, pneumonia, ear infections and permanent vision loss.
“In recent months, we’ve seen how swiftly and easily measles can make a comeback in communities where not enough children have been immunized,” said WHO Regional Director for the Western Pacific Takeshi Kasai.
Nine countries and areas in the Region have been verified as having eliminated measles: Australia, Brunei Darussalam, Cambodia, Hong Kong SAR (China), Japan, Macao SAR (China), New Zealand, the Republic of Korea and Singapore. Elimination means there has been no prolonged local transmission of the virus for at least three years.
But even in countries where measles has been eliminated, as long as the virus is circulating elsewhere, people who are not immunized remain at risk of infection from an imported case. This, in turn can lead to an outbreak or re-establishment of transmission.
So far this year, Australia, Cambodia, China, Hong Kong SAR (China), Japan, the Lao People’s Democratic Republic, New Zealand, the Republic of Korea, Singapore and Viet Nam have all recorded measles cases.
“The resurgence of measles around the world has resulted in increased importation of the virus to several countries in our Region,” explained Dr Kasai. “What we want to stop is large-scale outbreaks resulting from those importations.”
Threat of outbreaks continues
Everybody should be vaccinated in all countries, whether or not they have achieved elimination, according to WHO guidelines. For a community to be protected, at least 95% of children must receive two doses of measles vaccine.
“Measles spreads like wildfire,” Dr Kasai explained. “It is the most contagious human disease, and it’s very good at seeking out and spreading among even small groups of people who are not immune.”
Since 2000, more than 21 million lives have been saved worldwide through measles immunization. In 2017 in the Western Pacific Region, 97% of children received a first dose of measles vaccine (compared to 85% in 2000), and 94% got the required second dose (compared to 2% in 2000).
Need to reach unvaccinated children
Although every country in the Region has committed to eliminating measles, some populations are still missed by immunization programmes. The lack of national strategies and efforts to increase access to vaccination are part of the problem, especially in hard-to-reach areas. Misconceptions about the effectiveness or safety of vaccines can also complicate efforts to vaccinate children.
WHO supports countries across the Region in efforts to vaccinate all children and strengthen their outbreak preparation and response. The Organization also encourages countries to address the reasons why children are not being vaccinated in some communities by combating misinformation and improving understanding of the importance and safety of vaccines.
In 2018, WHO published a Regional Strategy and Plan of Action for Measles and Rubella Elimination in the Western Pacific. The Plan assists countries in strengthening immunization programmes and developing national action plans for achieving the shared goal of measles elimination.
The Red Cross has received permission from Venezuela’s government and opposition to roll out one of the organization’s biggest global relief campaigns
Friday, March 29th, 2019Dhaka, Bangladesh: The high-rise building where fire and smoke killed 25 people, did not have a proper fire suppression system — and some of its emergency exit doors were locked
Friday, March 29th, 20193/29/1982: The combination of an earthquake and a volcanic eruption at El Chichon in southern Mexico converts a hill into a crater, kills thousands of people and destroys acres of farmland
Friday, March 29th, 2019March 28, 1979, the worst accident in the history of the U.S. nuclear power industry begins when a pressure valve in the Unit-2 reactor at Three Mile Island fails to close and the core began to dangerously overheat.
Thursday, March 28th, 2019Migrants hijack the ship that rescued them when the captain made clear that he would return them to Libya
Thursday, March 28th, 2019“………A recent crackdown on crossings from Libya has led to a backlog of would-be migrants. Thousands of migrants remain in Libyan government-run detention centers, and nightmare accounts of forced labor, exploitation and inhumane conditions at the hands of the men they paid to deliver them across the Mediterranean have emerged…..”

