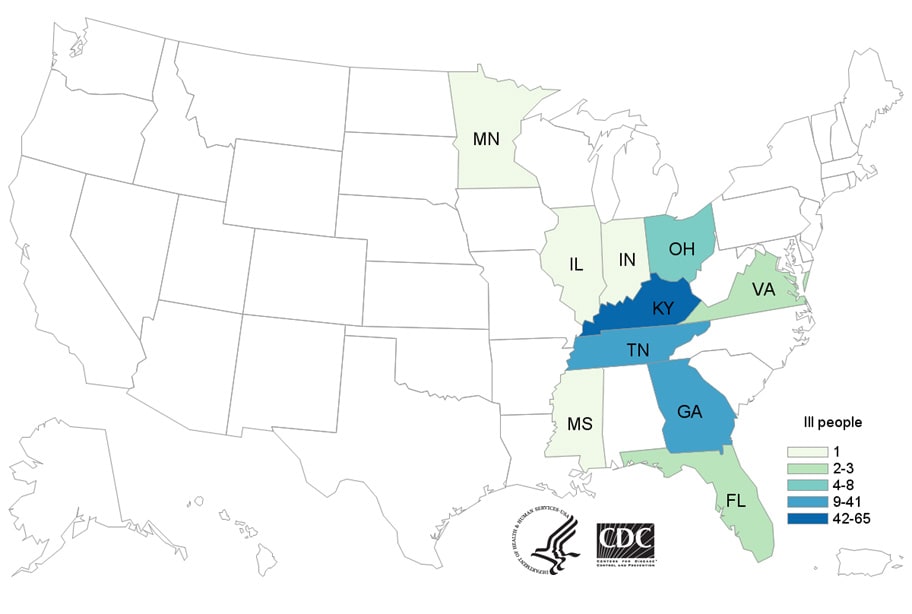
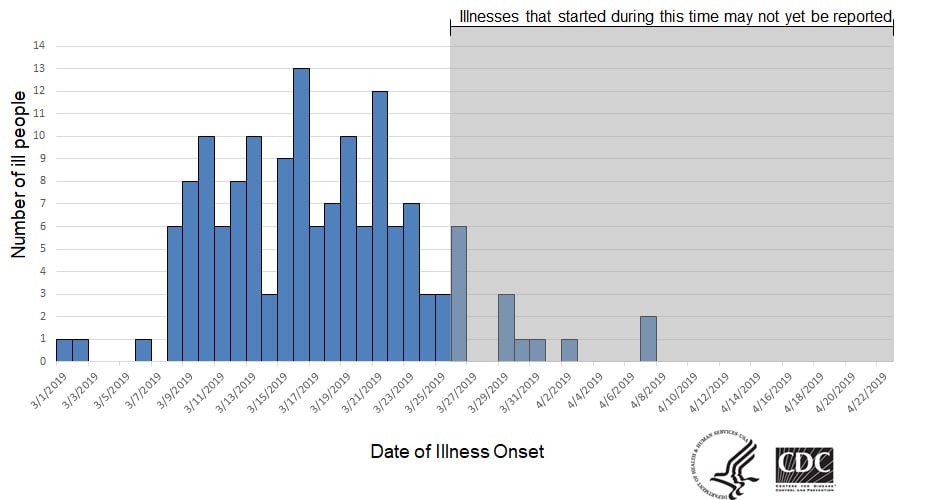
WHO welcomes the Government of Malawi’s launch of the world’s first malaria vaccine today in a landmark pilot programme. The country is the first of three in Africa in which the vaccine, known as RTS,S, will be made available to children up to 2 years of age; Ghana and Kenya will introduce the vaccine in the coming weeks.
Malaria remains one of the world’s leading killers, claiming the life of one child every two minutes. Most of these deaths are in Africa, where more than 250 000 children die from the disease every year. Children under 5 are at greatest risk of its life-threatening complications. Worldwide, malaria kills 435 000 people a year, most of them children.
“We have seen tremendous gains from bed nets and other measures to control malaria in the last 15 years, but progress has stalled and even reversed in some areas. We need new solutions to get the malaria response back on track, and this vaccine gives us a promising tool to get there,” said WHO Director-General Dr Tedros Adhanom Ghebreyesus. “The malaria vaccine has the potential to save tens of thousands of children’s lives.”
An innovation milestone, three decades in development
Thirty years in the making, RTS,S is the first, and to date the only, vaccine that has demonstrated it can significantly reduce malaria in children. In clinical trials, the vaccine was found to prevent approximately 4 in 10 malaria cases, including 3 in 10 cases of life-threatening severe malaria
“Malaria is a constant threat in the African communities where this vaccine will be given. The poorest children suffer the most and are at highest risk of death,” said Dr Matshidiso Moeti, WHO Regional Director for Africa. “We know the power of vaccines to prevent killer diseases and reach children, including those who may not have immediate access to the doctors, nurses and health facilities they need to save them when severe illness comes.”
“This is a day to celebrate as we begin to learn more about what this tool can do to change the trajectory of malaria through childhood vaccination,” she added.
The pilot programme is designed to generate evidence and experience to inform WHO policy recommendations on the broader use of the RTS,S malaria vaccine. It will look at reductions in child deaths; vaccine uptake, including whether parents bring their children on time for the four required doses; and vaccine safety in the context of routine use.
The vaccine is a complementary malaria control tool – to be added to the core package of WHO-recommended measures for malaria prevention, including the routine use of insecticide-treated bed nets, indoor spraying with insecticides, and the timely use of malaria testing and treatment.
A model public-private partnership
The WHO-coordinated pilot programme is a collaborative effort with ministries of health in Ghana, Kenya and Malawi and a range of in-country and international partners, including PATH, a non-profit organization, and GSK, the vaccine developer and manufacturer, which is donating up to 10 million vaccine doses for this pilot.
“We salute WHO and Malawi for their leadership in realizing this historic milestone,” said Steve Davis, President and CEO of PATH, “and we look forward to the start of vaccination in Ghana, and then Kenya later this year. A vaccine for malaria is among many innovations needed to bring an end to this disease, and we proudly stand with all countries and our many partners in progressing towards a malaria-free world.”
The malaria vaccine pilot aims to reach about 360,000 children per year across the three countries. Ministries of health will determine where the vaccine will be given; they will focus on areas with moderate-to-high malaria transmission, where the vaccine can have the greatest impact.
“Delivering the world’s first malaria vaccine will help reduce the burden of one of the most pressing health challenges globally. This novel tool is the result of GSK employees collaborating with their partners, applying the latest in vaccine science to contribute to the fight against malaria,” said Dr Thomas Breuer, Chief Medical Officer of GSK Vaccines. “We look forward to seeing the results of the pilot, and in parallel, are working with WHO and PATH to secure the vaccine’s sustained global health impact in the future.”
Financing and support
Financing for the pilot programme has been mobilized through an unprecedented collaboration among three key global health funding bodies: Gavi, the Vaccine Alliance; the Global Fund to Fight AIDS, Tuberculosis and Malaria; and Unitaid. Additionally, WHO, PATH and GSK are providing in-kind contributions.
Partner quotes
Dr Seth Berkley, CEO of Gavi
“Malaria is still one of the biggest killers of children worldwide, taking the lives of over 200,000 children every year. These pilots will be crucial to determine the part this vaccine could play in reducing the burden this disease continues to place on the world’s poorest countries.”
Lelio Marmora, Executive Director of Unitaid
“The malaria vaccine is an exciting innovation that complements the global health community’s efforts to end the malaria epidemic. It is also a shining example of the kind of inter-agency coordination that we need. We look forward to learning how the vaccine can be integrated for greatest impact into our work.”
Peter Sands, Executive Director of the Global Fund
“To step up the fight against malaria, we need every available tool. If this pilot shows that RTS,S is a cost-effective tool against malaria, it will help us save more children’s lives.”
Notes to the editors:
- Pilot countries: Following a request by WHO for expressions of interest, the pilot countries were selected from among 10 African countries. Key criteria for selection included well-functioning malaria and immunization programmes, and areas with moderate to high malaria transmission.
- Proven results: In Phase 3 trials conducted in Africa, between 2009 and 2014, children receiving 4 doses of RTS,S experienced significant reductions in malaria and malaria-related complications, in comparison to those who did not receive RTS,S. The vaccine prevented 4 in 10 cases of clinical malaria; 3 in 10 cases of severe malaria; and 6 in 10 cases of severe malaria anaemia, the most common reason children die from malaria. Significant reductions were also seen in overall hospital admissions and the need for blood transfusions, which are required to treat severe malaria anaemia. These and other benefits were in addition to those already seen through the use of insecticide-treated nets (bed nets); prompt diagnosis; and effective antimalarial treatment.
- Child vaccination schedule: In selected areas in the three countries, the vaccine will be given in 4 doses: 3 doses between 5 and 9 months of age and the fourth dose provided around the 2nd birthday.