Emergent BioSolutions today announced that it was awarded a contract worth about $2 billion over the next 10 years to deliver the ACAM200 smallpox vaccine to the Strategic National Stockpile.
September 4th, 2019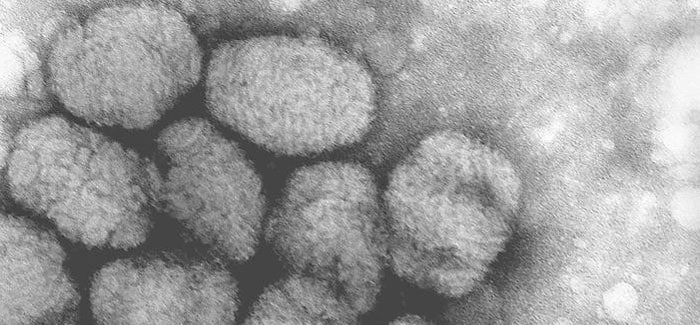
“…….About ACAM2000 Vaccine
ACAM2000 vaccine is the primary smallpox vaccine designated for use in a bioterrorism emergency, with almost 269 million doses having been supplied to the U.S. Strategic National Stockpile. ACAM2000 vaccine is also licensed in Australia and Singapore and is currently stockpiled both in the U.S. and internationally.
ACAM2000 is indicated for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection.
The labeling for ACAM2000 contains a contraindication for individuals with severe immunodeficiency. Severe localized or systemic infection with vaccinia (progressive vaccinia) may occur in persons with weakened immune systems. Individuals with severe immunodeficiency who are not expected to benefit from the vaccine should not receive ACAM2000. The risk for experiencing severe vaccination complications must be weighed against the risk for experiencing a potentially fatal smallpox infection.
Additionally, there are warnings and precautions for myocarditis, pericarditis, encephalitis, encephalomyelitis, encephalopathy, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson Syndrome), eczema vaccinatum resulting in permanent sequelae or death, ocular complications; blindness and fetal death have occurred following either primary vaccination or revaccination with live vaccinia virus smallpox vaccines. These risks are increased in certain individuals and may result in severe disability, permanent neurological sequalae and/or death.
Please see full Prescribing Information for full boxed warning and additional safety information…..”

