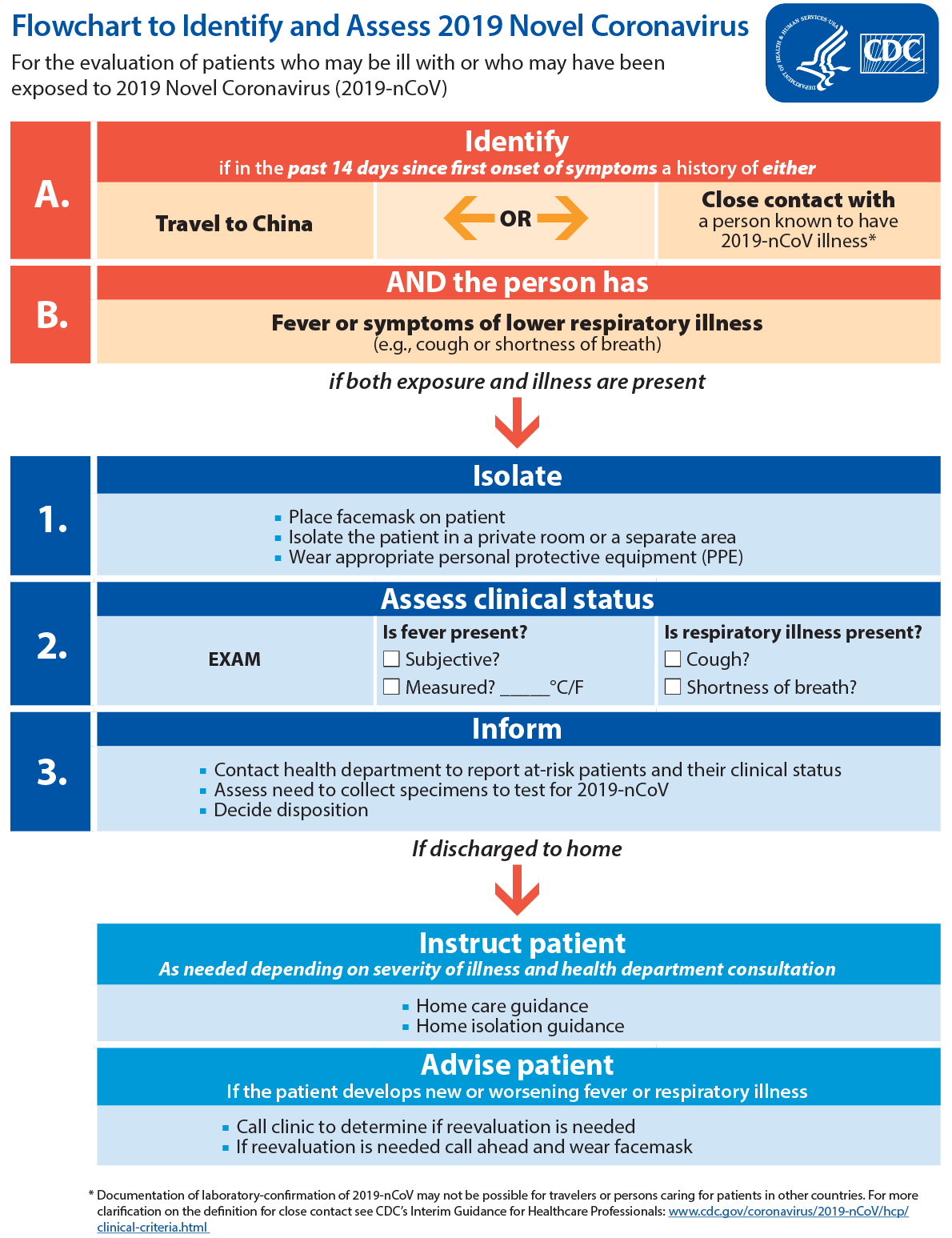
https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html
Background
CDC is closely monitoring an outbreak of respiratory illness caused by a novel (new) coronavirus (named by the World Health Organization as “2019-nCoV”) that was first detected in Wuhan, Hubei Province, China and which continues to expand. Chinese health officials have reported tens of thousands of infections with 2019-nCoV in China, with the virus spreading from person-to-person in many parts of that country. Transmission to healthcare personnel (HCP) in China has also been reported in the media; however, there is not yet detailed information about those transmission events. Infections with 2019-nCoV, most of them associated with travel from Wuhan, are also being reported from a growing number of international locations, including the United States. The first confirmed instance of person-to-person spread of 2019-nCoV in the United States was reported on January 30, 2020.
Much is unknown about 2019-nCoV. Current knowledge is largely based on what is known about similar coronaviruses. Coronaviruses are a large family of viruses that are common in humans and in many different species of animals, including camels, cattle, cats, and bats. Rarely, animal coronaviruses can infect people and then spread between people such as with SARS-CoV, MERS-CoV, and likely now with 2019-nCoV.
Early reports suggest spread from person-to-person most likely happens during close exposure to a person infected with 2019-nCoV. Person-to-person spread may occur similar to other coronaviruses, mainly via respiratory droplets produced when an infected person coughs. These droplets can land in the mouths, noses, or eyes of people who are nearby or possibly be inhaled into the lungs. Currently, the extent to which touching a surface or object that has the virus on it and then touching their own mouth, nose, or possibly their eyes, contributes to transmission is unclear.
Purpose
This interim guidance is intended to assist with assessment of risk, monitoring, and work restriction decisions for HCP with potential exposure to 2019-nCoV. For guidance on assessment and management of exposure risk in non-healthcare settings, refer to the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings. The guidance for non-healthcare settings can also be used to identify the movement, public activity and travel restrictions that apply to the HCP included here.
Because of their often extensive and close contact with vulnerable individuals in healthcare settings, a conservative approach to HCP monitoring and restriction from work was taken to quickly identify early symptoms and prevent transmission from potentially contagious HCP to patients, HCP, and others visiting or working in a healthcare setting. For this reason, the signs and symptoms* described in this guidance are more inclusive than those described when assessing exposures for individuals not working in healthcare. Healthcare facilities should have a low threshold for evaluating symptoms and testing symptomatic HCP, particularly those who fall into the high- and medium-risk categories described in this guidance.
This guidance is based on the currently limited information available about 2019-nCoV. Based on these uncertainties, the recommendations regarding which HCP are restricted from work may not prevent all transmission or anticipate every potential scenario, and will change if indicated by new information.
Healthcare facilities, in consultation with public health authorities should use clinical judgement as well as the principles outlined in this guidance to assign risk and determine need for work restrictions. CDC remains available for further consultation by calling the Emergency Operations Center at 770-488-7100. This cautious approach will be refined and updated as more information becomes available and as response needs change in the United States.
Other Resources
For guidance on risk assessment and public health management of persons not working in a U.S. healthcare setting refer to: Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings.
For infection prevention and control guidance for healthcare settings caring for Persons with Known or Under Investigation (PUI) for 2019 Novel Coronavirus (2019-nCoV), refer to the Interim Infection Prevention and Control Recommendations for Patients with Known or Persons Under Investigation for 2019 Novel Coronavirus (2019-nCoV) in a Healthcare Setting.
I. Definitions Used in this Guidance
Self-monitoring means HCP should monitor themselves for fever by taking their temperature twice a day and remain alert for respiratory symptoms (e.g., cough, shortness of breath, sore throat)*. Anyone on self-monitoring should be provided a plan for whom to contact if they develop fever or respiratory symptoms during the self-monitoring period to determine whether medical evaluation is needed.
Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat)*. For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. The mode of communication can be determined by the state or local public health authority and may include telephone calls or any electronic or internet-based means of communication.
For HCP, active monitoring can be delegated by the health department to the HCP’s healthcare facility occupational health or infection control program, if both the health department and the facility are in agreement. Note, inter-jurisdictional coordination will be needed if HCP live in a different local health jurisdiction than where the healthcare facility is located.
Self-Monitoring with delegated supervision in a healthcare setting means HCP perform self-monitoring with oversight by their healthcare facility’s occupational health or infection control program in coordination with the health department of jurisdiction, if both the health department and the facility are in agreement. Occupational health or infection control personnel should establish points of contact between the organization, the self-monitoring personnel, and the local or state health departments of authority in the location where self-monitoring personnel will be during the self-monitoring period. This communication should result in agreement on a plan for medical evaluation of personnel who develop fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat)* during the self-monitoring period. The plan should include instructions for notifying occupational health and the local public health authority, and transportation arrangements to a designated hospital, if medically necessary, with advance notice if fever or respiratory symptoms occur. The supervising organization should remain in contact with HCP through the self-monitoring period to oversee self-monitoring activities. Note, inter-jurisdictional coordination will be needed if HCP live in a different local health jurisdiction than where the healthcare facility is located.
Close contact for healthcare exposures is defined as follows: a) being within approximately 6 feet (2 meters), of a person with 2019-nCoV infection for a prolonged period of time (such as caring for or visiting the patient; or sitting within 6 feet of the patient in a healthcare waiting area or room); or b) having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand).
Data to inform the definition of close contact are limited. Considerations when assessing close contact include the duration of exposure (e.g., longer exposure time likely increases exposure risk), clinical symptoms of the patient (e.g., coughing likely increases exposure risk) and whether the patient was wearing a facemask (which can efficiently block respiratory secretions from contaminating others and the environment). It is not possible to define the duration of time that constitutes a prolonged exposure. However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1 to 2 minutes) exposure as prolonged.
Currently brief interactions are considered to be less likely to result in transmission; however, as described above, this is dependent on the clinical symptoms of the patient and type of interaction (e.g., did the patient cough directly into the face of the HCP). Information about this will be updated as more information becomes available. Risk stratification can be made in consultation with public health authorities. Examples of brief interactions include: briefly entering the patient room without having direct contact with the patient or their secretions/excretions, brief conversation at a triage desk with a patient who was not wearing a facemask. See Table 1 for more detailed information.
Healthcare Personnel: For the purposes of this document HCP refers to refers to all paid and unpaid persons serving in healthcare settings who have the potential for direct or indirect exposure to patients or infectious materials, including body substances; contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air. For this document, HCP does not include clinical laboratory personnel.
II. Defining Exposure Risk Category
While body fluids other than respiratory secretions have not been clearly implicated in transmission of 2019-nCoV, unprotected contact with other body fluids, including blood, stool, vomit, and urine, should also be considered as potentially putting HCP at risk of 2019-nCoV infection, until further data are available.
When assigning risk, factors to consider include: the duration of exposure (e.g., longer exposure time likely increases exposure risk), clinical symptoms of the patient (e.g., coughing likely increases exposure risk), whether the patient was wearing a facemask (which can efficiently block respiratory secretions from contaminating others and the environment), whether an aerosol generating procedure was performed, and the type of PPE used by HCP. However, data on the risk of transmission of 2019-nCoV are currently incomplete and the precision of current risk assignment is limited. Table 1 describes possible scenarios that can be used to assist with risk assessment. These scenarios do not cover all potential exposure scenarios and should not replace an individual assessment of risk for the purpose of clinical decision making or individualized public health management. Any public health decisions that place restrictions on an individual’s or group’s movements or impose specific monitoring requirements should be based on an assessment of risk for the individual or group. Healthcare facilities, in consultation with public health authorities should use the concepts outlined in this guidance along with clinical judgement to assign risk and determine need for work restrictions.
For this guidance high-risk exposures refer to HCP who performed or were present in the room for procedures that generate aerosols or during which respiratory secretions are likely to be poorly controlled (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) on patients with 2019-nCoV when the healthcare providers’ eyes, nose, or mouth were not protected.
Medium-risk exposures generally include HCP who had prolonged close contact with patients with 2019-nCoV where HCP mucous membranes or hands were exposed to material potentially infectious with 2019-nCoV. These exposures could place the exposed HCP at risk of developing disease that is less than that described under high-risk.
Proper adherence to currently recommended infection control practices, including all recommended PPE, should protect HCP having prolonged close contact with patients infected with 2019-nCoV. However, HCP in this category are classified as having low-risk to account for any inconsistencies in use or adherence that could result in unrecognized exposures.
HCP with no direct patient contact and no entry into active patient management areas who adhere to routine safety precautions are not considered to have a risk of exposure to 2019-nCoV (i.e., they have no identifiable risk.)
Currently the guidance is intended to apply to HCP with potential exposure in a healthcare setting to patients with confirmed 2019-nCoV infection. However, HCP exposures will commonly involve a PUI who is awaiting testing. Implementation of the monitoring and work restrictions described in this guidance could be applied to HCP exposed to a PUI if test results for the PUI are not expected to return within 48 to 72 hours. A record of HCP exposed to the PUI should still be maintained and HCP should be encouraged to perform self- monitoring while awaiting test results. If the results will be delayed more than 72 hours or the patient is positive for 2019-nCoV then all monitoring and work restrictions described in this document should be followed.
Table 1: Epidemiologic Risk Classification1 for Asymptomatic Healthcare Personnel Following Exposure to Patients with 2019 Novel Coronavirus (2019-nCoV) Infection or their Secretions/Excretions in a Healthcare Setting, and their Associated Monitoring and Work Restriction Recommendations
The distinction between the high- and medium-risk exposures in this document is somewhat artificial as they both place HCP at risk for developing infection; therefore the recommendations for active monitoring and work restrictions are the same for these exposures. However, these risk categories were created to align with risk categories described in the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings, which outlines criteria for quarantine and travel restrictions specific to high-risk exposures. Refer to that Interim Guidance for information about the movement, public activity and travel restrictions that apply to the HCP included here.
The highest risk exposure category that applies should be used to guide monitoring and work restrictions.
Table 1: Epidemiologic Risk Classification1 for Asymptomatic Healthcare Personnel Following Exposure to Patients with 2019 Novel Coronavirus (2019-nCoV) Infection or their Secretions/Excretions in a Healthcare Setting, and their Associated Monitoring and Work Restriction Recommendations
| Epidemiologic risk factors |
Exposure category |
Recommended Monitoring for 2019-nCoV (until 14 days after last potential exposure) |
Work Restrictions for Asymptomatic HCP |
| A. HCP (with unprotected eyes, nose, or mouth) who perform or are present in the room for a procedure likely to generate higher concentrations of respiratory secretions or aerosols (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) |
High |
Active |
Exclude from work for 14 days after last exposure |
| B. HCP who perform or are present in the room for a procedure likely to generate higher concentrations of respiratory secretions or aerosols (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) and not using a gown and gloves.Note: If the HCP’s eyes, nose, or mouth were also unprotected they would fall into the high-risk category above. |
Medium |
Active |
Exclude from work for 14 days after last exposure |
| C. HCP (with unprotected eyes, nose, or mouth) who have prolonged close contact with a patient who was not wearing a facemaskNote: A respirator confers a higher level of protection than a facemask. However, they are group together in this scenario because (even if a respirator or facemask was worn) the eyes remain uncovered while having prolonged close contact with a patient who was not wearing a facemask. |
Medium |
Active |
Exclude from work for 14 days after last exposure |
| D. HCP (with unprotected eye, nose, and mouth) who have prolonged close contact with a patient who was wearing a facemask |
Medium |
Active |
Exclude from work for 14 days after last exposure |
| E. HCP (not wearing gloves) who have direct contact with the secretions/excretions of a patient and the HCP failed to perform immediate hand hygieneNote: If the HCP performed hand hygiene immediately after contact, this would be considered low risk. |
Medium |
Active |
Exclude from work for 14 days after last exposure |
| F. HCP wearing a facemask or respirator only who have prolonged close contact with a patient who was wearing a facemaskNote: A respirator confers a higher level of protection than a facemask. However, they are grouped together in this scenario and classified as low-risk because the patient was wearing a facemask for source control. |
Low |
Self with delegated supervision |
None |
| G. HCP using all recommended PPE (i.e., a respirator, eye protection, gloves and a gown) while caring for or having contact with the secretions/excretions of a patient |
Low |
Self with delegated supervision |
None |
| H. HCP (not using all recommended PPE) who have brief interactions with a or patient regardless of whether patient was wearing a facemask (e.g., brief conversation at a triage desk; briefly entering a patient room but not having direct contact with the patient or their secretions/excretions; entering the patient room immediately after they have been discharged) |
Low |
Self with delegated supervision |
None |
| I. HCP who walk by a patient or who have no direct contact with the patient or their secretions/excretions and no entry into the patient room |
No identifiable risk |
None |
None |
HCP=healthcare personnel; PPE=personal protective equipment
1 The distinction between the high- and medium-risk exposures in this document is somewhat artificial as they both place HCP at risk for developing infection and the recommendations for active monitoring and work restrictions are the same for these exposures. However, these risk categories were created to align with risk categories described in the Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (2019-nCoV) Exposure in Travel-associated or Community Settings, which outlines criteria for quarantine and travel restrictions specific to high-risk exposures. Refer to that Interim Guidance for information about the movement, public activity and travel restrictions that apply to the HCP included here.
III. Recommendations for Monitoring Based on 2019-nCoV Exposure Risk
HCP in any of the risk exposure categories who develop signs or symptoms compatible with 2019-nCoV infection must contact their established point of contact (public health authorities or their facility’s occupational health program) for medical evaluation prior to returning to work
-
High- and Medium-risk Exposure Category
HCP in the high- or medium-risk category should undergo active monitoring, including restriction from work in any healthcare setting until 14 days after their last exposure. If they develop any fever (measured temperature >100.0oF or subjective fever) OR respiratory symptoms consistent with 2019-nCoV infection (e.g., cough, shortness of breath, sore throat)* they should immediately self-isolate (separate themselves from others) and notify their local or state public health authority and healthcare facility promptly so that they can coordinate consultation and referral to a healthcare provider for further evaluation.
-
Low-risk Exposure Category
HCP in the low-risk category should perform self-monitoring with delegated supervision until 14 days after the last potential exposure. Asymptomatic HCP in this category are not restricted from work. They should check their temperature twice daily and remain alert for respiratory symptoms consistent with 2019-nCoV infection infection (e.g., cough, shortness of breath, sore throat)*. They should ensure they are afebrile and asymptomatic before leaving home and reporting for work. If they do not have fever or respiratory symptoms they may report to work. They should have their temperature retaken and symptoms assessed by the healthcare facility each day before starting work. On days they are not working they are not required to report unless they develop symptoms. If they develop fever (measured temperature > 100.0 oF or subjective fever) OR respiratory symptoms they should immediately self-isolate (separate themselves from others) and notify their local or state public health authority or healthcare facility promptly so that they can coordinate consultation and referral to a healthcare provider for further evaluation.
-
No Identifiable risk Exposure Category
HCP in the no identifiable risk category do not require monitoring or restriction from work.
-
Community or travel-associated exposures
HCP with potential exposures to 2019-nCoV in community settings, should have their exposure risk assessed according CDC guidance. HCP who fall into the high- or medium- risk category described there should undergo monitoring as defined by their local or state public health authority and be excluded from work in a healthcare setting until 14 days after their exposure. HCP who develop signs or symptoms compatible with 2019-nCoV should contact their established point of contact (public health authorities or their facility’s occupational health program) for medical evaluation prior to returning to work.
*Fever is either measured temperature >100.0oF or subjective fever. Note that fever may be intermittent or may not be present in some patients, such as those who are elderly, immunosuppressed, or taking certain medications (e.g., NSAIDs). Clinical judgement should be used to guide testing of patients in such situations. Respiratory symptoms consistent with 2019-nCoV infection are cough, shortness of breath, and sore throat. Medical evaluation may be recommended for lower temperatures (<100.0oF) or other symptoms (e.g., muscle aches, nausea, vomiting, diarrhea, abdominal pain headache, runny nose, fatigue) based on assessment by public health authorities.