Archive for the ‘CDC’ Category
CDC’s Zika MAC test has been cleared by FDA (Emergency Use Authorization) for detecting evidence of the virus in human sera or CSF
Saturday, February 27th, 2016** the Zika MAC test is used to gauge recent infection and can detect the virus as early as 4 days after symptoms begin.
“….. the Food and Drug Administration (FDA) issue an Emergency Use Authorization (EUA) for emergency use of the Centers for Disease Control and Prevention’s (CDC) Zika Immunoglobulin M (IgM) Antibody Capture Enzyme-Linked Immunosorbent Assay (Zika MAC-ELISA) for the presumptive detection of Zika virusspecific IgM in human sera or cerebrospinal fluid (CSF)……”
** The overall effectiveness of this year’s flu vaccine is 59%. Getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%
Friday, February 26th, 2016As of February 23, 2016, CDC and state public health departments are investigating 14 additional reports of possible sexual transmission of the Zika virus
Wednesday, February 24th, 2016
Update: Interim Guidelines for Prevention of Sexual Transmission of Zika Virus — United States, 2016
Distributed via the CDC Health Alert Network
February 23, 2016, 14:15 EST (2:15 PM EST)
CDCHAN-00388
Summary: The Centers for Disease Control and Prevention (CDC) recently published recommendations for protecting people against sexual transmission of Zika virus (1). As stated in that report, information about possible sexual transmission of Zika virus was based on one published report of transmission from a man to a woman, one published report in which Zika virus was detected in semen of a man with hematospermia, and one case of possible sexual transmission then under investigation in Texas. An additional case of Zika virus detected in semen in a man was reported after the CDC recommendations were published (2). As of February 23, 2016, CDC and state public health departments are investigating 14 additional reports of possible sexual transmission of the virus, including several involving pregnant women. While additional investigations are being completed, CDC is issuing this HAN Advisory as a strong reminder to state, local, and US territorial public health departments, clinicians, and the public to be aware of and adhere to current recommendations for preventing sexual transmission of Zika virus, particularly for men with pregnant partners. These recommendations may change as more information becomes available.
Background
CDC is working with state, local, and US territorial public health departments, US Government agencies, and international partners in response to outbreaks of Zika virus disease (Zika) in multiple territories and countries in the Americas. Accumulating evidence links maternal Zika virus infection with congenital microcephaly, miscarriages, and other adverse fetal outcomes (3). In addition, there are reports of a possible association with Guillain-Barré syndrome (4). No vaccine or specific antiviral drug is currently available to prevent or treat Zika.
Zika virus is spread primarily by the bite of infected Aedes species mosquitoes (most commonly, Aedes aegypti). In areas where Zika virus transmission is ongoing, people should follow precautions to prevent mosquito bites (http://www.cdc.gov/zika/prevention/). Sexual transmission of Zika virus also can occur and is of particular concern during pregnancy. In early February 2016, the Dallas County Department of Health and Human Services announced an occurrence of sexually transmitted Zika infection (5). On February 5, 2016, following the confirmation of this Texas sexual transmission event, CDC published interim guidelines for preventing sexual transmission of Zika virus (1).
As of February 23, 2016, CDC and state public health departments are investigating 14 additional reports of possible sexual transmission of the virus, including several events involving possible transmission to pregnant women. In two of these new suspected sexual transmission events that have been investigated to date, Zika virus infection has been confirmed in women whose only known risk factor was sexual contact with an ill male partner who had recently travelled to an area with local Zika virus transmission; testing for the male partners is pending. For four additional suspected sexual transmission events, preliminary laboratory evidence (IgM antibody test) is available for the women, but confirmatory testing is still pending. For eight suspected events, the investigation is ongoing. In all events for which information is available, travelers reported symptom onset within 2 weeks prior to their non-traveling female partner’s symptom onset.
Because these reports suggest sexual transmission may be a more likely means of transmission for Zika virus than previously considered, CDC is issuing this HAN Advisory to underscore the importance of adhering to the interim guidance published on February 5 and outlined below. The recommendations, which apply to men who reside in or have traveled to areas with active Zika virus transmission (http://wwwnc.cdc.gov/travel/notices/) and their sex partners, will be revised as more information becomes available.
Recommendations for men and their pregnant partners
Men who reside in or have traveled to an area of active Zika virus transmission who have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex (i.e., vaginal intercourse, anal intercourse, or fellatio) for the duration of the pregnancy. Pregnant women should discuss their male partner’s potential exposures to mosquitoes and history of Zika-like illness (http://www.cdc.gov/zika/symptoms) with their health care provider; providers can consult CDC’s guidelines for evaluation and testing of pregnant women (6).
Recommendations for men and their nonpregnant sex partners
Men who reside in or have traveled to an area of active Zika virus transmission who are concerned about sexual transmission of Zika virus might consider abstaining from sexual activity or using condoms consistently and correctly during sex. Couples considering this personal decision should take several factors into account. Most infections are asymptomatic, and when illness does occur, it is usually mild with symptoms lasting from several days to a week; severe disease requiring hospitalization is uncommon. The risk for acquiring vector-borne Zika virus in areas of active transmission depends on the duration and extent of exposure to infected mosquitoes and the steps taken to prevent mosquito bites (http://www.cdc.gov/zika/prevention). After infection, Zika virus might persist in semen when it is no longer detectable in blood; studies to determine the duration of persistence in semen are not yet completed.
Accumulating evidence of sexual transmission suggests that exposure to Zika virus includes unprotected sexual contact with a symptomatic male partner who resides in or has traveled to an area of active Zika virus transmission. Zika virus testing is currently recommended to establish a diagnosis of infection in exposed persons with signs or symptoms consistent with Zika virus disease, and may be offered to asymptomatic pregnant women with possible exposure to Zika virus (6). However, interpretation of results is complex, and health care providers should contact their state, local, or territorial health department for assistance with arranging testing and interpreting results. At this time, testing of exposed, asymptomatic men for the purpose of assessing risk for sexual transmission is not recommended. Sexual transmission of Zika virus from infected women to their sex partners has not been documented, nor has transmission from persons who are asymptomatically infected. Sexual transmission of many infections, including those caused by other viruses, is reduced by consistent and correct use of latex condoms.
As we learn more about the incidence and duration of seminal shedding from infected men and the utility and availability of testing in this context, recommendations to prevent sexual transmission of Zika virus will be updated.
References
- Oster AM, Brooks JT, Stryker JE, et al. Interim Guidelines for prevention of sexual transmission of Zika virus — United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:120–121. http://www.cdc.gov/mmwr/volumes/65/wr/mm6505e1.htm
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen [letter]. Emerg Infect Dis. 2016 May [cited February 22, 2016].http://dx.doi.org/10.3201/eid2205.160107
- Martines RB, Bhatnagar J, Keating MK, et al. Evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses — Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65 (Early Release)(06):1-2. http://www.cdc.gov/mmwr/volumes/65/wr/mm6506e1.htm?s_cid=mm6506e1_e. Published February 19, 2016.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome – 10 December 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf. Published 2015. Accessed Feb 1, 2016.
- Dallas County Health and Human Services. DCHHS reports first Zika virus case in Dallas County acquired through sexual transmission. February 2, 2016. http://www.dallascounty.org/department/hhs/press/documents/PR2-2-16DCHHSReportsFirstCaseofZikaVirusThroughSexualTransmission.pdf
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65.http://www.cdc.gov/mmwr/volumes/65/wr/mm6505e2.htm?s_cid=mm6505e2_e
For More Information
- General information about Zika virus and disease: http://www.cdc.gov/zika/
- Zika virus information for clinicians: http://www.cdc.gov/zika/hc-providers/index.html
- Protection against mosquitoes: http://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/protection-against-mosquitoes-ticks-other-arthropods
- Travel notices related to Zika virus: http://wwwnc.cdc.gov/travel/notices
- Information about Zika virus for travelers and travel health providers: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/zika
- HAN Advisory: Recognizing, managing, and reporting Zika virus infections in travelers returning from Central America, South America, the Caribbean, and Mexico. January 15, 2016. http://emergency.cdc.gov/han/han00385.asp
- Pan American Health Organization PAHO): http://www.paho.org/hq/index.php?option=com_content&view=article&id=11585&Itemid=41688〈=en
- Approximate distribution of Aedes aegypti and Ae. albopictus mosquitoes in the United States:
http://www.cdc.gov/chikungunya/resources/vector-control.html
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and controlling diseases and injuries; enhances health decisions by providing credible information on critical health issues; and promotes healthy living through strong partnerships with local, national and international organizations.
DEPARTMENT OF HEALTH AND HUMAN SERVICES
HAN Message Types
- Health Alert: Conveys the highest level of importance; warrants immediate action or attention. Example: HAN00001
- Health Advisory: Provides important information for a specific incident or situation; may not require immediate action. Example: HAN00346
- Health Update: Provides updated information regarding an incident or situation; unlikely to require immediate action. Example: HAN00342
- Info Service: Provides general information that is not necessarily considered to be of an emergent nature. Example: HAN00345
U.S. and Brazilian researchers to recruit mothers and babies in one of the biggest government-led studies to understand whether the Zika virus is linked to microcephaly
Sunday, February 21st, 2016“…..Researchers hope to enroll 100 mothers and their babies with microcephaly. These will be matched with 300 to 400 pairs of healthy mothers and their babies. Initial results are expected this spring……..
Blood samples from moms and babies in both groups will be tested for signs of Zika infection.
Current diagnostic tests looking for Zika antibodies are limited because they closely resemble dengue, a related virus common in Brazil. The team hopes that taking samples from both mothers and their babies will give a more precise picture of whether Zika was involved. They will also look for other exposures that might explain why a baby developed microcephaly.
The study’s design should help determine the relative risk of microcephaly in babies whose mothers were infected with Zika.
Staples said the work could provide stronger confirmation of a link, but that it would take years of scientific investigation to prove whether Zika actually causes microcephaly…..”
Interim Guidelines for Prevention of Sexual Transmission of Zika Virus — United States, 2016
Tuesday, February 9th, 2016Interim Guidelines for Prevention of Sexual Transmission of Zika Virus — United States, 2016
Early Release / February 5, 2016 / 65(5);1–2
Alexandra M. Oster, MD1; John T. Brooks, MD1; Jo Ellen Stryker, PhD1; Rachel E. Kachur2; Paul Mead, MD3; Nicki T. Pesik, MD4; Lyle R. Petersen, MD3
Zika virus is a mosquito-borne flavivirus primarily transmitted by Aedes aegypti mosquitoes (1,2). Infection with Zika virus is asymptomatic in an estimated 80% of cases (2,3), and when Zika virus does cause illness, symptoms are generally mild and self-limited. Recent evidence suggests a possible association between maternal Zika virus infection and adverse fetal outcomes, such as congenital microcephaly (4,5), as well as a possible association with Guillain-Barré syndrome. Currently, no vaccine or medication exists to prevent or treat Zika virus infection. Persons residing in or traveling to areas of active Zika virus transmission should take steps to prevent Zika virus infection through prevention of mosquito bites (http://www.cdc.gov/zika/prevention/).
Sexual transmission of Zika virus is possible, and is of particular concern during pregnancy.
Current information about possible sexual transmission of Zika is based on reports of three cases.
The first was probable sexual transmission of Zika virus from a man to a woman (6), in which sexual contact occurred a few days before the man’s symptom onset. The second is a case of sexual transmission currently under investigation (unpublished data, 2016, Dallas County Health and Human Services). The third is a single report of replication-competent Zika virus isolated from semen at least 2 weeks and possibly up to 10 weeks after illness onset; reverse transcriptase-polymerase chain reaction testing of blood plasma specimens collected at the same time as the semen specimens did not detect Zika virus (7). The man had no sexual contacts. Because no further testing was conducted, the duration of persistence of Zika virus in semen remains unknown.
In all three cases, the men developed symptomatic illness. Whether infected men who never develop symptoms can transmit Zika virus to their sex partners is unknown. Sexual transmission of Zika virus from infected women to their sex partners has not been reported. Sexual transmission of many infections, including those caused by other viruses, is reduced by consistent and correct use of latex condoms.
The following recommendations, which apply to men who reside in or have traveled to areas with active Zika virus transmission (http://wwwnc.cdc.gov/travel/notices/) and their sex partners, will be revised as more information becomes available.
Recommendations for men and their pregnant partners
Men who reside in or have traveled to an area of active Zika virus transmission who have a pregnant partner should abstain from sexual activity or consistently and correctly use condoms during sex (i.e., vaginal intercourse, anal intercourse, or fellatio) for the duration of the pregnancy. Pregnant women should discuss their male partner’s potential exposures to mosquitoes and history of Zika-like illness (http://www.cdc.gov/zika/symptoms) with their health care provider; providers can consult CDC’s guidelines for evaluation and testing of pregnant women (8).
Recommendations for men and their nonpregnant sex partners
Men who reside in or have traveled to an area of active Zika virus transmission who are concerned about sexual transmission of Zika virus might consider abstaining from sexual activity or using condoms consistently and correctly during sex. Couples considering this personal decision should take several factors into account. Most infections are asymptomatic, and when illness does occur, it is usually mild with symptoms lasting from several days to a week; severe disease requiring hospitalization is uncommon. The risk for acquiring vector-borne Zika virus in areas of active transmission depends on the duration and extent of exposure to infected mosquitoes and the steps taken to prevent mosquito bites (http://www.cdc.gov/zika/prevention). After infection, Zika virus might persist in semen when it is no longer detectable in blood.
Zika virus testing has been recommended to establish a diagnosis of infection in some groups, such as pregnant women (8). At present, Zika virus testing for the assessment of risk for sexual transmission is of uncertain value, because current understanding of the incidence and duration of shedding in the male genitourinary tract is limited to one case report in which Zika virus persisted longer than in blood (7). At this time, testing of men for the purpose of assessing risk for sexual transmission is not recommended. As we learn more about the incidence and duration of seminal shedding from infected men and the utility and availability of testing in this context, recommendations to prevent sexual transmission of Zika virus will be updated.
References
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009;15:1347–50. CrossRef PubMed
- CDC. Zika virus. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. http://www.cdc.gov/zika/index.html.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360:2536–43. CrossRef PubMed
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2015. http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016;47:6–7. CrossRef PubMed
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011;17:880–2. CrossRef PubMed
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015;21:359–61. CrossRef PubMed
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65.
Suggested citation for this article: Oster AM, Brooks JT, Stryker JE, et al. Interim Guidelines for Prevention of Sexual Transmission of Zika Virus — United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65(Early Release):1–2. DOI: http://dx.doi.org/10.15585/mmwr.mm6505e1er.
CDC Update: Interim Guidelines for Health Care Providers Caring for Pregnant Women and Women of Reproductive Age with Possible Zika Virus Exposure — United States, 2016
Sunday, February 7th, 2016
Update: Interim Guidelines for Health Care Providers Caring for Pregnant Women and Women of Reproductive Age with Possible Zika Virus Exposure — United States, 2016
Early Release / February 5, 2016 / 65(05);1–6
Titilope Oduyebo, MD1,2; Emily E. Petersen, MD2; Sonja A. Rasmussen, MD3; Paul S. Mead, MD4; Dana Meaney-Delman, MD5; Christina M. Renquist, MPH6; Sascha R. Ellington, MSPH2; Marc Fischer, MD4; J. Erin Staples, MD, PhD4; Ann M. Powers, PhD4; Julie Villanueva, PhD4; Romeo R. Galang, MD1,7; Ada Dieke, DrPH1,2; Jorge L. Muñoz, PhD4; Margaret A. Honein, PhD6; Denise J. Jamieson, MD2
CDC has updated its interim guidelines for U.S. health care providers caring for pregnant women during a Zika virus outbreak (1). Updated guidelines include a new recommendation to offer serologic testing to asymptomatic pregnant women (women who do not report clinical illness consistent with Zika virus disease) who have traveled to areas with ongoing Zika virus transmission. Testing can be offered 2–12 weeks after pregnant women return from travel. This update also expands guidance to women who reside in areas with ongoing Zika virus transmission, and includes recommendations for screening, testing, and management of pregnant women and recommendations for counseling women of reproductive age (15–44 years). Pregnant women who reside in areas with ongoing Zika virus transmission have an ongoing risk for infection throughout their pregnancy. For pregnant women with clinical illness consistent with Zika virus disease,* testing is recommended during the first week of illness. For asymptomatic pregnant women residing in areas with ongoing Zika virus transmission, testing is recommended at the initiation of prenatal care with follow-up testing mid-second trimester. Local health officials should determine when to implement testing of asymptomatic pregnant women based on information about levels of Zika virus transmission and laboratory capacity. Health care providers should discuss reproductive life plans, including pregnancy intention and timing, with women of reproductive age in the context of the potential risks associated with Zika virus infection.
Zika virus is primarily transmitted by Aedes aegypti mosquitoes, which are found throughout much of the region of the Americas, including parts of the United States (2,3). These mosquitoes can also transmit dengue and chikungunya viruses (4). The Zika virus outbreak continues to spread (http://www.cdc.gov/zika/geo/index.html), with ongoing Zika virus transmission recently reported in U.S. territories. Evidence suggesting an association of Zika virus infection with an increased risk for congenital microcephaly and other abnormalities of the brain and eye (5) prompted the World Health Organization to declare the Zika virus outbreak a Public Health Emergency of International Concern on February 1, 2016 (http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/).
There is currently no vaccine or medication to prevent Zika virus infection. All travelers to or residents of areas with ongoing Zika virus transmission should be advised to strictly follow steps to avoid mosquito bites because of the potential for exposure to Zika, dengue, and chikungunya viruses (6). Aedes vector mosquitoes bite mostly during daylight hours; thus, protection from mosquito bites is required throughout the day (7). Prevention of mosquito bites includes wearing long-sleeved shirts, pants, permethrin-treated clothing, and using United States Environmental Protection Agency (EPA)-registered insect repellents. Insect repellents containing ingredients such as DEET, picaridin, and IR3535 are safe for use during pregnancy when used in accordance with the product label (6). To prevent human-to-mosquito-to-human transmission, persons infected with Zika, dengue, or chikungunya virus should protect themselves from mosquito exposure during the first week of illness. The number of mosquitoes in and around homes can be reduced by emptying standing water from containers, installing or repairing screens on windows and doors, and using air conditioning if available. Further information on preventing mosquito bites is available online (http://www.cdc.gov/features/stopmosquitoes/).
Antiviral treatment is not currently available for Zika virus disease; treatment is supportive and includes rest, fluids, and analgesic and antipyretic medications. Aspirin and other nonsteroidal anti-inflammatory medications should be avoided until dengue virus infection can be ruled out (8). Dengue virus infection can cause serious complications, including hemorrhage and death, which might be substantially reduced by early recognition and supportive treatment (4,8). Pregnant women with fever should be treated with acetaminophen (9).
Updated Recommendations for Testing Pregnant Women with a History of Travel to Areas with Ongoing Zika Virus Transmission
Recommendations for Zika virus testing of pregnant women who have a clinical illness consistent with Zika virus disease during or within 2 weeks of travel to areas with ongoing Zika virus transmission are unchanged from CDC recommendations released January 19, 2016 (1). Zika virus testing of maternal serum includes reverse transcription-polymerase chain reaction (RT-PCR) testing for symptomatic patients with onset of symptoms during the previous week; immunoglobulin M (IgM) and plaque-reduction neutralizing antibody testing should be performed on specimens collected ≥4 days after onset of symptoms (Figure 1) (1,10).
Serologic testing for Zika virus can be offered to asymptomatic pregnant women who traveled to an area with ongoing Zika virus transmission (Figure 1); however, interpretation of results is complex. Because of cross-reactivity among related flaviviruses, such as dengue, yellow fever, and West Nile viruses, a positive IgM result can be difficult to interpret. Plaque-reduction neutralization testing (PRNT) can be performed to measure virus-specific neutralizing antibodies to Zika virus and other flaviviruses. The levels of neutralizing antibodies can then be compared between flaviviruses, but these tests might also be difficult to interpret in persons who were previously infected with or vaccinated against flaviviruses. However, a negative IgM result obtained 2–12 weeks after travel would suggest that a recent infection did not occur and could obviate the need for serial ultrasounds. Based on experience with other flaviviruses, IgM antibodies will be expected to be present at least 2 weeks after virus exposure and persist for up to 12 weeks (11–14). Information about the performance of serologic testing of asymptomatic persons is limited; a negative serologic test result obtained 2–12 weeks after travel cannot definitively rule out Zika virus infection. Given these challenges in interpreting serologic test results, health care providers should contact their state, local, or territorial health department for assistance with arranging testing and interpreting results. CDC is working with health departments and other organizations to rapidly increase the availability of testing for Zika virus.
Guidelines for Pregnant Women Residing in Areas with Ongoing Zika Virus Transmission
Pregnant women who reside in areas with ongoing Zika virus transmission should be evaluated for symptoms of Zika virus disease. For women who report clinical illness consistent with Zika virus disease, testing by RT-PCR should be performed on serum collected within 7 days of symptom onset. Because viremia decreases over time, a negative RT-PCR result from serum collected 5–7 days after symptom onset does not exclude Zika virus infection, and serologic testing should be performed. (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf).
A false positive IgM result is more likely among women residing in areas with ongoing Zika virus transmission than among travelers because of a higher likelihood of previous exposure to a related flavivirus. Pregnant women who do not report clinical illness consistent with Zika virus disease can be offered IgM testing upon initiation of prenatal care; among women with negative IgM results, repeat testing can be considered in the mid-second trimester because of the ongoing risk for Zika virus exposure and infection throughout pregnancy (Figure 2).
Pregnant women with negative Zika virus IgM testing should receive routine prenatal care, including an assessment of pregnancy dating and an ultrasound at 18–20 weeks of gestation to assess fetal anatomy (15). The ultrasound should include careful evaluation of the fetus for brain anomalies, including microcephaly and intracranial calcifications. Because fetal microcephaly is most easily detected in the late second and early third trimesters of pregnancy (16), and because of ongoing potential exposure to Zika virus, health care providers might consider an additional fetal ultrasound later in pregnancy.
Findings of fetal microcephaly or intracranial calcifications on prenatal ultrasound should prompt health care providers to repeat maternal IgM testing and consider amniocentesis, depending on gestational age. Zika virus testing can be performed on amniotic fluid using RT-PCR to inform clinical management (5). Based on experience with other congenital infections and a small number of prenatally-diagnosed fetal Zika virus infections (5,17), amniocentesis can be used to diagnose intrauterine infections (18). However, the performance of RT-PCR testing of amniotic fluid for Zika virus infection has not been evaluated. Furthermore, the risk for microcephaly or other anomalies when Zika virus RNA is detected in amniotic fluid is not known.
Serial fetal ultrasounds should be considered to monitor fetal anatomy and growth every 3–4 weeks in pregnant women with positive or inconclusive Zika virus test results, and referral to a maternal-fetal medicine specialist is recommended. Testing is recommended at the time of delivery, including histopathologic examination of the placenta and umbilical cord, testing of frozen placental tissue and cord tissue for Zika virus RNA, and testing of cord serum (1,19). Guidelines for infants whose mothers have possible Zika virus infection are available (19). If a pregnant woman with Zika virus disease experiences a fetal loss, Zika virus RT-PCR and immunohistochemical staining should be performed on fetal tissues, including umbilical cord and placenta (1).
Sexual transmission of Zika virus can occur, although there is limited data about the risk (20). The risk for sexual transmission of Zika virus can be eliminated by abstinence and reduced by correct and consistent use of condoms (21). Given the potential risks of maternal Zika virus infection, pregnant women whose male partners have or are at risk for Zika virus infection should consider using condoms or abstaining from sexual intercourse (21). Additional studies are needed to characterize the risk for sexual transmission of Zika virus; recommendations will be updated as more information becomes available.
Special Considerations for Women of Reproductive Age Residing in Areas of Ongoing Zika Virus Transmission
CDC recommends that health care providers discuss pregnancy intention and reproductive options with women of reproductive age. Decisions regarding the timing of pregnancies are personal and complex; reproductive life plans can assist in making these decisions (22). Patient age, fertility, reproductive and medical history, as well as the values and preferences of the woman and her partner should be considered during discussions regarding pregnancy intentions and timing. In the context of the ongoing Zika virus transmission, preconception care should include a discussion of the signs and symptoms and the potential risks associated with Zika virus infection.
Health care providers should discuss strategies to prevent unintended pregnancy with women who do not want to become pregnant; these strategies should include counseling on family planning and use of contraceptive methods. Safety, effectiveness, availability, and acceptability should be considered when selecting a contraceptive method (23). Approximately half of U.S. pregnancies each year are unintended (24); patients should be counseled to use the most effective contraceptive method that can be used correctly and consistently. For women desiring highly effective contraception, long acting reversible contraception, including contraceptive implants and intrauterine devices, might be the best choice (http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/PDF/Contraceptive_methods_508.pdf). When choosing a contraceptive method, the risk for sexually transmitted infections should also be considered; correct and consistent use of condoms reduces the risk for sexually transmitted infections.
Strategies to prevent mosquito bites should be emphasized for women living in areas with ongoing Zika virus transmission who want to become pregnant. These strategies, including wearing pants and long-sleeved shirts, using FDA-approved insect repellents, ensuring that windows and doors have screens, and staying inside air conditioned spaces when possible, can reduce the risk for Zika virus infection and other vector-borne diseases. During preconception counseling visits, the potential risks of Zika virus infection acquired during pregnancy should be discussed.
Women of reproductive age with current or previous laboratory-confirmed Zika virus infection should be counseled that there is no evidence that prior Zika virus infection poses a risk for birth defects in future pregnancies (7). This is because the viremia is expected to last approximately 1 week in patients with clinical illness (2,25). There is no current evidence to suggest that a fetus conceived after maternal viremia has resolved would be at risk for fetal infection (7).
References
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:30–3. CrossRef PubMed
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009;15:1347–50. CrossRef PubMed
- CDC. Chikungunya virus. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html.
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva, Switzerland: World Health Organization; 2009. http://apps.who.int/iris/bitstream/10665/44188/1/9789241547871_eng.pdf.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016;47:6–7. CrossRef PubMed
- CDC. West Nile virus: insect repellent use & safety. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. http://www.cdc.gov/westnile/faq/repellent.html.
- CDC. Zika virus. Atlanta, GA: US Department of Health and Human Services, CDC; 2015. http://www.cdc.gov/zika/index.html.
- CDC. Dengue virus. Atlanta, GA: US Department of Health and Human Services, CDC; 2009. http://www.cdc.gov/Dengue/.
- Rasmussen SA, Kissin DM, Yeung LF, et al. ; Pandemic Influenza and Pregnancy Working Group. Preparing for influenza after 2009 H1N1: special considerations for pregnant women and newborns. Am J Obstet Gynecol 2011;204(Suppl 1):S13–20. CrossRef PubMed
- Division of Vector-Borne Diseases. Arboviral Diseases and Dengue Branches. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf.
- Babaliche P, Doshi D. Catching dengue early: clinical features and laboratory markers of dengue virus infection. J Assoc Physicians India 2015;63:38–41. PubMed
- Wahala WMPB, de Silva AM. The human antibody response to dengue virus infection. Viruses 2011;3:2374–95. CrossRef PubMed
- Gibney KB, Edupuganti S, Panella AJ, et al. Detection of anti-yellow fever virus immunoglobulin m antibodies at 3–4 years following yellow fever vaccination. Am J Trop Med Hyg 2012;87:1112–5. CrossRef PubMed
- Roehrig JT, Nash D, Maldin B, et al. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed west nile virus encephalitis cases. Emerg Infect Dis 2003;9:376–9. CrossRef PubMed
- American Academy of Pediatrics/American College of Obstetricians and Gynecologists. Guidelines for perinatal care. 7th ed. Elk Grove Village, IL: American Academy of Pediatrics/American College of Obstetricians and Gynecologists; 2012.
- Bromley B, Benacerraf BR. Difficulties in the prenatal diagnosis of microcephaly. J Ultrasound Med 1995;14:303–6. PubMed
- American College of Obstetricians and Gynecologists. Practice bulletin no. 151: Cytomegalovirus, parvovirus B19, varicella zoster, and toxoplasmosis in pregnancy. Obstet Gynecol 2015;125:1510–25. CrossRef PubMed
- Picone O, Costa JM, Leruez-Ville M, Ernault P, Olivi M, Ville Y. Cytomegalovirus (CMV) glycoprotein B genotype and CMV DNA load in the amniotic fluid of infected fetuses. Prenat Diagn 2004;24:1001–6. CrossRef PubMed
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7. CrossRef PubMed
- Foy BD, Kobylinski KC, Foy JLC, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011;17:880–2. CrossRef PubMed
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 2015;65(5).
- CDC. Reproductive life plan tool for health professionals. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. http://www.cdc.gov/preconception/rlptool.html.
- Division of Reproductive Health. National Center for Chronic Disease Prevention. U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013;62(RR-05).
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014;104(Suppl 1):S43–8. CrossRef PubMed
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232–9. CrossRef PubMed
* Clinical illness consistent with Zika virus disease is defined as two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis.
 FIGURE 1. Updated Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman with history of travel to an area with ongoing Zika virus transmission
FIGURE 1. Updated Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman with history of travel to an area with ongoing Zika virus transmission
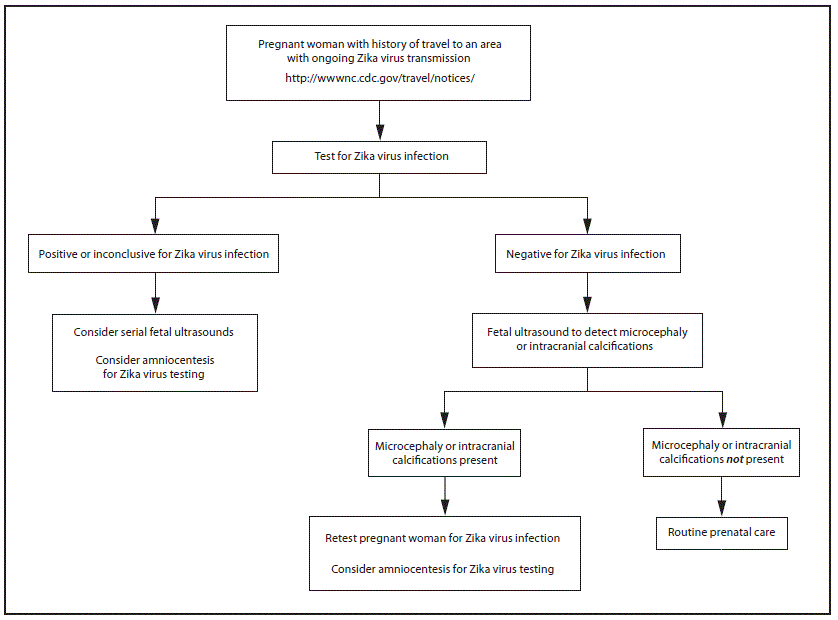 * Testing is recommended for pregnant women with clinical illness consistent with Zika virus disease, which includes two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis during or within 2 weeks of travel. Testing includes Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended.
* Testing is recommended for pregnant women with clinical illness consistent with Zika virus disease, which includes two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis during or within 2 weeks of travel. Testing includes Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended.
† Testing can be offered to pregnant women without clinical illness consistent with Zika virus disease. If performed, testing should include Zika virus IgM, and if IgM test result is positive or indeterminate, neutralizing antibodies on serum specimens. Testing should be performed 2–12 weeks after travel.
§ Laboratory evidence of maternal Zika virus infection: 1) Zika virus RNA detected by RT-PCR in any clinical specimen; or 2) positive Zika virus IgM with confirmatory neutralizing antibody titers that are ≥4-fold higher than dengue virus neutralizing antibody titers in serum. Testing is considered inconclusive if Zika virus neutralizing antibody titers are <4-fold higher than dengue virus neutralizing antibody titers.
¶ Fetal ultrasounds might not detect microcephaly or intracranial calcifications until the late second or early third trimester of pregnancy.
** Amniocentesis is not recommended until after 15 weeks of gestation. Amniotic fluid should be tested for Zika virus RNA by RT-PCR. The sensitivity and specificity of RT-PCR testing on amniotic fluid are not known.
 FIGURE 2. Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman residing in an area with ongoing Zika virus transmission,†† with or without clinical illness consistent with Zika virus disease§§
FIGURE 2. Interim guidance: testing algorithm*,†,§,¶,** for a pregnant woman residing in an area with ongoing Zika virus transmission,†† with or without clinical illness consistent with Zika virus disease§§
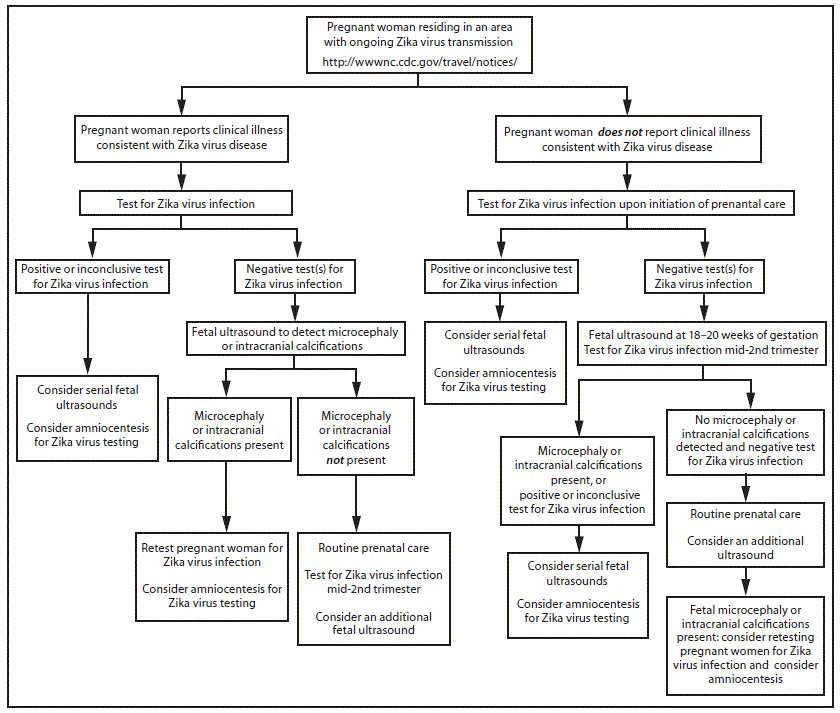 * Tests for pregnant women with clinical illness consistent with Zika virus disease include Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended. If chikungunya or dengue virus RNA is detected, treat in accordance with existing guidelines. Timely recognition and supportive treatment for dengue virus infections can substantially lower the risk of medical complications and death. Repeat Zika virus testing during pregnancy is warranted if clinical illness consistent with Zika virus disease develops later in pregnancy.
* Tests for pregnant women with clinical illness consistent with Zika virus disease include Zika virus reverse transcription-polymerase chain reaction (RT-PCR), and Zika virus immunoglobulin M (IgM) and neutralizing antibodies on serum specimens (http://www.aphl.org/Materials/CDCMemo_Zika_Chik_Deng_Testing_011916.pdf). Because of the overlap of symptoms and areas where other viral illnesses are endemic, evaluation for dengue or chikungunya virus infection is also recommended. If chikungunya or dengue virus RNA is detected, treat in accordance with existing guidelines. Timely recognition and supportive treatment for dengue virus infections can substantially lower the risk of medical complications and death. Repeat Zika virus testing during pregnancy is warranted if clinical illness consistent with Zika virus disease develops later in pregnancy.
† Testing can be offered to pregnant women without clinical illness consistent with Zika virus disease. If performed, testing should include Zika virus IgM, and if IgM test result is positive or indeterminate, neutralizing antibodies on serum specimens. Results from serologic testing are challenging to interpret in areas where residents have had previous exposure to other flaviviruses (e.g., dengue, yellow fever).
§ Laboratory evidence of maternal Zika virus infection: 1) Zika virus RNA detected by RT-PCR in any clinical specimen; or 2) positive Zika virus IgM with confirmatory neutralizing antibody titers that are ≥4-fold higher than dengue virus neutralizing antibody titers in serum. Testing is considered inconclusive if Zika virus neutralizing antibody titers are <4-fold higher than dengue virus neutralizing antibody titer.
¶ Amniocentesis is not recommended until after 15 weeks gestation. Amniotic fluid should be tested for Zika virus RNA by RT-PCR. The sensitivity and specificity of RT-PCR testing on amniotic fluid are not known.
** Fetal ultrasounds might not detect microcephaly or intracranial calcifications until the late second or early third trimester of pregnancy.
†† Local health officials should determine when to implement testing of asymptomatic pregnant women based on information about levels of Zika virus transmission and laboratory capacity.
§§ Clinical illness consistent with Zika virus disease is defined as two or more of the following signs or symptoms: acute onset of fever, maculopapular rash, arthralgia, or conjunctivitis.
Suggested citation for this article: Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: Interim Guidelines for Health Care Providers Caring for Pregnant Women and Women of Reproductive Age with Possible Zika Virus Exposure — United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65(Early Release):1–6. DOI: http://dx.doi.org/10.15585/mmwr.mm6505e2er.
CDC adds 4 destinations to interim travel guidance related to Zika virus: American Samoa, Costa Rica, Curacao, and Nicaragua
Tuesday, February 2nd, 2016
CDC is working with other public health officials to monitor for ongoing Zika virus transmission. Today, CDC added the following destinations to the Zika virus travel alerts: American Samoa, Costa Rica, Curacao, and Nicaragua. CDC has issued a travel alert (Level 2-Practice Enhanced Precautions) for people traveling to regions and certain countries where Zika virus transmission is ongoing. For a full list of affected countries/regions: http://www.cdc.gov/zika/geo/index.html. Specific areas where Zika virus transmission is ongoing are often difficult to determine and are likely to continue to change over time.
As more information becomes available, CDC travel alerts will be updated. Travelers to areas where cases of Zika virus infection have been recently confirmed are at risk of being infected with the Zika virus. Mosquitoes that spread Zika are aggressive daytime biters, prefer to bite people, and live indoors and outdoors near people. There is no vaccine or medicine available for Zika virus. The best way to avoid Zika virus infection is to prevent mosquito bites.
Some travelers to areas with ongoing Zika virus transmission will become infected while traveling but will not become sick until they return home. Some people who are infected do not have any symptoms. Symptoms include fever, rash, joint pain, and red eyes. Other commonly reported symptoms include muscle pain and headache. The illness is usually mild with symptoms lasting from several days to a week. Severe disease requiring hospitalization is uncommon and case fatality is low. Travelers to these areas should monitor for symptoms or illness upon return. If they become ill, they should tell their healthcare professional where they have traveled and when.
Until more is known, CDC continues to recommend that pregnant women and women trying to become pregnant take the following precautions:
- Pregnant women should consider postponing travel to the areas where Zika virus transmission is ongoing. Pregnant women who must travel to one of these areas should talk to their doctor or other healthcare professional first and strictly follow steps to avoid mosquito bites during the trip.
- Women trying to become pregnant should consult with their healthcare professional before traveling to these areas and strictly follow steps to prevent mosquito bites during the trip.
Guillain-Barré syndrome (GBS) has been reported in patients with probable Zika virus infection in French Polynesia and Brazil. Because we do not know if Zika virus infection causes GBS, research efforts are underway to examine if there is a potential link between Zika and GBS.
###
CDC Health Advisory: Flu Season Begins — Severe Influenza Illness Reported
Tuesday, February 2nd, 2016
|
||
CDC: Interim Guidelines for The Evaluation and Testing of Infants with Possible Congenital Zika Virus Infection — United States, 2016
Wednesday, January 27th, 2016Interim Guidelines for the Evaluation and Testing of Infants with Possible Congenital Zika Virus Infection — United States, 2016
CDC has developed interim guidelines for health care providers in the United States who are caring for infants born to mothers who traveled to or resided in an area with Zika virus transmission during pregnancy. These guidelines include recommendations for the testing and management of these infants. Guidance is subject to change as more information becomes available; the latest information, including answers to commonly asked questions, can be found online (http://www.cdc.gov/zika). Pediatric health care providers should work closely with obstetric providers to identify infants whose mothers were potentially infected with Zika virus during pregnancy (based on travel to or residence in an area with Zika virus transmission [http://wwwnc.cdc.gov/travel/notices]), and review fetal ultrasounds and maternal testing for Zika virus infection (see Interim Guidelines for Pregnant Women During a Zika Virus Outbreak*) (1). Zika virus testing is recommended for 1) infants with microcephaly or intracranial calcifications born to women who traveled to or resided in an area with Zika virus transmission while pregnant; or 2) infants born to mothers with positive or inconclusive test results for Zika virus infection. For infants with laboratory evidence of a possible congenital Zika virus infection, additional clinical evaluation and follow-up is recommended. Health care providers should contact their state or territorial health department to facilitate testing. As an arboviral disease, Zika virus disease is a nationally notifiable condition.
Zika virus is a mosquito-borne flavivirus primarily transmitted by Aedes aegypti mosquitoes (2,3). Aedes albopictus mosquitoes also might transmit the virus. Ae. aegypti and Ae. albopictus mosquitoes are found throughout much of the Region of the Americas, including parts of the United States, and also transmit dengue and chikungunya viruses (4). Zika virus infections have also been documented through both intrauterine transmission resulting in congenital infection and intrapartum transmission from a viremic mother to her newborn (5,6). Zika virus RNA has been detected in breast milk, but Zika virus transmission through breastfeeding has not been documented (5).
During outbreaks, humans are the primary host for Zika virus. An estimated 80% of persons infected with Zika virus are asymptomatic (2,7). Symptomatic disease generally is mild and characterized by acute onset of fever, maculopapular rash, arthralgia, or nonpurulent conjunctivitis. Symptoms typically last from several days to 1 week. Based on information from previous outbreaks, severe disease requiring hospitalization is uncommon and fatalities are rare (6,7). During the current outbreak in Brazil, Zika virus RNA has been identified in specimens (i.e., brain tissue, placenta, and amniotic fluid) from several infants with microcephaly and from fetal losses in women infected with Zika virus during pregnancy (6,8,9). The Brazil Ministry of Health has reported a marked increase from previous years in the number of infants born with microcephaly and intracranial calcifications in 2015, although it is not known how many of these cases are associated with Zika virus infection (6,8–11).
Zika Virus Testing Considerations and Classification
The diagnosis of Zika virus infection is made through molecular and serologic testing (2). This includes reverse transcription-polymerase chain reaction (RT-PCR) for viral RNA, and immunoglobulin (Ig) M ELISA and plaque reduction neutralization test (PRNT) for Zika virus antibodies. Because it is currently not known which type of testing most reliably establishes the diagnosis of congenital infection, CDC recommends both molecular and serologic testing of infants who are being evaluated for evidence of a congenital Zika virus infection (Box 1). No commercial tests for Zika virus are available; Zika virus testing is performed at CDC and some state and territorial health departments. Health care providers should contact their state or territorial health department to facilitate testing.
Zika virus RT-PCR testing should be performed on serum specimens collected from the umbilical cord or directly from the infant within 2 days of birth (12). In addition, cerebrospinal fluid (CSF) obtained for other studies, and frozen and fixed placenta obtained at delivery, should also be tested by RT-PCR. IgM ELISA for Zika virus and dengue virus should be performed on infant serum, infant CSF, and maternal serum; however, results of these assays can be falsely positive because of cross-reacting antibodies (9,12). PRNT can be performed to measure virus-specific neutralizing antibodies and to discriminate between cross-reacting antibodies from closely related flaviviruses (e.g., dengue or yellow fever viruses). Finally, immunohistochemical staining to detect Zika virus antigen on fixed placenta and umbilical cord tissues can be considered.
An infant is considered congenitally infected if Zika virus RNA or viral antigen is identified in any of the samples submitted, including testing of amniotic fluid and testing of the placenta or umbilical cord. In addition, Zika virus IgM antibodies with confirmatory neutralizing antibody titers that are ≥4-fold higher than dengue virus neutralizing antibody titers in the infant serum or CSF constitute evidence of a congenital Zika virus infection. If Zika virus neutralizing antibody titers are <4-fold higher than dengue, results are considered inconclusive.
Recommendations for Infants with Microcephaly or Intracranial Calcifications Detected Prenatally or at Birth Whose Mothers Were Potentially Infected with Zika Virus During Pregnancy
For the purpose of evaluating an infant for possible congenital Zika virus infection, microcephaly is defined as occipitofrontal circumference less than the third percentile, based on standard growth charts (e.g., Fenton, Olsen, CDC, or WHO growth curves) for sex, age, and gestational age at birth (13). For a diagnosis of microcephaly to be made, the occipitofrontal circumference should be disproportionately small in comparison with the length of the infant and not explained by other etiologies (e.g., other congenital disorders). If an infant’s occipitofrontal circumference is equal to or greater than the third percentile but is notably disproportionate to the length of the infant, or if the infant has deficits that are related to the central nervous system, additional evaluation for Zika virus infection might be considered.
When an infant is born with microcephaly or intracranial calcifications to a mother who was potentially infected with Zika virus during pregnancy, the infant should be tested for Zika virus infection (Figure 1) (Box 1). In addition, further clinical evaluation and laboratory testing is recommended for the infant (Box 2). The mother should also be tested for a Zika virus infection, if this testing has not already been performed during pregnancy. An ophthalmologic evaluation, including retinal examination, should occur during the first month of life, given reports of abnormal eye findings in infants with possible congenital Zika virus infection (11).
For infants with any positive or inconclusive test findings for Zika virus infection, health care providers should report the case to the state, territorial, or local health department and assess the infant for possible long-term sequelae (Box 3). This includes a repeat hearing screen at age 6 months, even if the initial hearing screening test was normal, because of the potential for delayed hearing loss as has been described with other infections such as cytomegalovirus (14).
For infants with microcephaly or intracranial calcifications who have negative results on all Zika virus tests performed, health care providers should evaluate for other possible etiologies and treat as indicated.
Recommendations for Infants without Microcephaly or Intracranial Calcifications Whose Mothers Were Potentially Infected with Zika Virus During Pregnancy
For an infant without microcephaly or intracranial calcifications born to a mother who was potentially infected with Zika virus during pregnancy, subsequent evaluation is dependent on results from maternal Zika virus testing (Figure 2). If the test results for the mother were negative for Zika virus infection, the infant should receive routine care (e.g., newborn metabolic and hearing screens). If the mother received positive or inconclusive results of tests for Zika virus infection, the infant should be tested for a possible congenital Zika virus infection (Box 1). If the results of all of the infant’s tests are negative for evidence of Zika virus infection, then no further Zika virus testing and evaluation is recommended. If any of the infant’s samples test positive or inconclusive, then the infant should undergo further clinical evaluation (Box 2). The infant should also be followed to assess for possible long-term sequelae (Box 3), and the infant’s case should be reported to the state, territorial, or local health department. Infant follow-up should include a cranial ultrasound to assess for subclinical findings, unless prenatal ultrasound results from the third trimester demonstrated no abnormalities of the brain. Ophthalmologic examination and a repeat hearing screen are also recommended, as previously described for infants with microcephaly or intracranial calcifications. Developmental monitoring and screening during the first year of life is recommended for all children with congenital Zika virus infection.
If the mother has not undergone any previous testing for Zika virus infection during pregnancy, CDC recommends that she receive testing only if she reported symptoms consistent with Zika virus disease during or within 2 weeks of any time spent in an area with ongoing Zika virus transmission while she was pregnant (1,15). If the mother has any positive or inconclusive findings from tests for Zika virus infection, then the infant should undergo testing for evidence of a congenital Zika virus infection (Box 1). If the mother has not received any previous testing for Zika virus, and did not report clinical illness consistent with Zika virus disease during pregnancy, no further testing of the mother or infant is recommended (Figure 2).
Management and Prevention of Congenital Zika Virus Infections
No specific antiviral treatment is available for Zika virus infections and no vaccine against Zika virus is available (2). Treatment of congenital Zika virus infection is supportive and should address specific medical and neurodevelopmental issues for the infant’s particular needs; investigations are ongoing to better understand what services will be most effective for these children as they grow (16). Mothers are encouraged to breastfeed infants even in areas where Zika virus is found, as available evidence indicates the benefits of breastfeeding outweigh any theoretical risks associated with Zika virus infection transmission through breast milk (5,17).
The only way to prevent congenital Zika virus infection is to prevent maternal infection, either by avoiding areas where Zika virus transmission is ongoing or strictly following steps to avoid mosquito bites (15,18). Mosquito-bite prevention includes using air conditioning or window and door screens when indoors, wearing long sleeves and pants, using permethrin-treated clothing and gear, and using insect repellents. When used according to the product label, U.S. Environmental Protection Agency-registered insect repellents are safe for pregnant women (18).
** WHO: The mosquito-borne Zika virus is expected to spread to all countries in the Americas except for Canada and Chile.
Monday, January 25th, 2016CDC: Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Possible Association Between Zika Virus Infection and Microcephaly — Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65(Early Release):1–4. DOI: http://dx.doi.org/10.15585/mmwr.mm6503e2er.
CDC: Hennessey M, Fischer M, Staples JE. Zika Virus Spreads to New Areas — Region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep 2016;65(Early Release):1–4. DOI: http://dx.doi.org/10.15585/mmwr.mm6503e1er.


