Archive for the ‘CDC’ Category
CDC’s Emergency Drugs for US Clinicians and Hospitals
Thursday, June 8th, 2017Our Formulary
The following information is provided as an informational resource for guidance only. It is not intended as a substitute for professional judgment. These highlights and any hyperlinks may not include all the information needed to use each respective drug or biologic safely and effectively. See full prescribing information (package insert) or IND protocol for each respective drug or biologic, which accompany the product when it is delivered to the treating physician and/or pharmacist.
The Drug Service formulary is subject to change based on current public health needs, updates to treatment guidelines, and/or drug availability. For historical reference, we have included products no longer supplied by the Drug Service.
| Product & Supplier | Indication & Eligibility | How Supplied |
|---|---|---|
| Anthrax Vaccine Absorbed
(Also known as “AVA”; BioThrax®, Emergent BioSolutions) |
For the active immunization for the prevention of disease caused by Bacillus anthracis, in persons 18 through 65 years of age at high risk for exposure
Because the risk for anthrax infection in the general population is low, routine immunization is not recommended The safety and efficacy of BioThrax® in a post-exposure setting have not been established. |
Suspension for injection in 5 mL multidose vials, each containing 10 doses |
| Artesunate, intravenous
(Supplied to CDC by the Walter Reed Army Institute of Research) |
For the treatment of severe malaria in patients who require parenteral (IV) therapy
Patient must meet the eligibility criteria in the IND protocol |
110 mg; sterile dry-filled powder with phosphate buffer diluent for reconstitution |
| Benznidazole
(Benznidazol, Manufactured by LAFEPE) |
For the treatment of American trypanosomiasis (Chagas disease)
Patient must meet the eligibility criteria in the IND protocol |
100 mg double-scored tablet
12.5 mg dispersible tablet for pediatric use |
| Botulism Antitoxin Heptavalent (Equine), Types A-G
(Also known as “HBAT”; Manufactured by Cangene Corp. – BAT™) |
For the treatment of symptomatic botulism following documented or suspected exposure to botulinum neurotoxin | 20 mL or 50 mL single-use glass vial
May be received frozen or thawed |
| Diethylcarbamazine
(Also known as “DEC”; Supplied to CDC by the World Health Organization; Manufactured by E.I.P.I.C.O.) |
For the treatment of certain filarial diseases, including lymphatic filariasis caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori; tropical pulmonary eosinophilia; and loiasis
For prophylactic use in persons determined to be at increased risk for Loa loa infection Patient must meet the eligibility criteria in the IND protocol |
100 mg tablet |
| Diphtheria Antitoxin (Equine)
(Also known as “DAT”; Manufactured by Instituto Butantan) |
For prevention or treatment of actual or suspected cases of diphtheria
Patient must meet the eligibility criteria in the IND protocol |
1 mL single-use ampule containing 10,000 units |
| Eflornithine
(Also known as “DFMO”; Supplied to CDC by the World Health Organization; Manufactured by Sanofi Aventis – Ornidyl®) |
For the treatment of second-stage African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei gambiense, with involvement of the central nervous system | 20 g/100 mL hypertonic solution for IV infusion
Must be diluted with Sterile Water for Injection before use |
| Melarsoprol
(Supplied to CDC by the World Health Organization; Manufactured by Sanofi Aventis – Arsobal®) |
For the treatment of second-stage African trypanosomiasis (sleeping sickness), with involvement of the central nervous system
Patient must meet the eligibility criteria in the IND protocol |
5 mL glass ampule containing 180 mg/5 mL (36 mg/mL) |
| Nifurtimox
(Supplied to CDC by the World Health Organization; Manufactured by Bayer – Lampit®) |
For the treatment of American trypanosomiasis (Chagas disease)
Patient must meet the eligibility criteria in the IND protocol |
120 mg double-scored tablet |
| Sodium Stibogluconate
(Manufactured by GlaxoSmithKline, UK – Pentostam®) |
For the treatment of leishmaniasis
Patient must meet the eligibility criteria in the IND protocol |
Solution for injection in 100 mL multidose bottle
100 mg pentavalent antimony (Sb) per mL |
| Suramin
(Supplied to CDC by the World Health Organization; Manufactured by Bayer – Germanin) |
For the treatment of first-stage African trypanosomiasis (sleeping sickness) caused by Trypanosoma brucei rhodesiense, without involvement of the central nervous system
Patient must meet the eligibility criteria in the IND protocol |
1 gram of suramin for injection in a 10 mL vial (100 mg/mL solution of suramin sodium)
Must be reconstituted with 10 mL Sterile Water for Injection before use |
| Vaccinia Vaccine
(Also known as the “Smallpox Vaccine”; Manufactured by Sanofi Aventis – ACAM2000®) |
For active immunization against smallpox disease for persons determined to be at high risk for smallpox infection | Lyophilized powder reconstituted with diluent (provided)
Contains 100 doses per vial |
Anthrax Vaccine Adsorbed
Anthrax Vaccine Adsorbed (AVA) is indicated for the active immunization for the prevention of disease caused by Bacillus anthracis, in persons 18 through 65 years of age at high risk for exposure. The safety and efficacy of AVA in a post-exposure setting have not been established.
CDC provides anthrax vaccine for laboratory workers conducting research under federally funded projects who require preexposure vaccination based on their occupational risk.
Preexposure vaccination is recommended for laboratorians at risk for repeated exposure to fully virulent B. anthracis spores, such as those who 1) work with high concentrations of spores with potential for aerosol production; 2) handle environmental samples that might contain powders and are associated with anthrax investigations; 3) routinely work with pure cultures of B. anthracis; 4) frequently work in spore-contaminated areas after a bioterrorism attack; or 5) work in other settings where repeated exposures to B. anthracis aerosols may occur. Read more[PDF – 36 pages](https://www.cdc.gov/mmwr/pdf/rr/rr5906.pdf).
More Information for Clinicians
CDC’s Anthrax Vaccination Website
Educational Toolkit for Clinicians (from Department of Defense Anthrax Immunization Program)
Full Prescribing Information for BioThrax®
How to Request
Anthrax vaccine must be administered by or under the supervision of the physician who registers with CDC.
Contact the CDC Drug Service for more information.
Artesunate, Intravenous
Artesunate is in the class of medications known as artemesinins, which are derivatives from the “qinghaosu” or sweet wormwood plant (Artemisia annua). Artesunate is not currently licensed by FDA but is made available in the United States under a CDC-sponsored IND protocol for treatment of documented cases of severe malaria(https://www.cdc.gov/malaria/about/index.html) that require parenteral therapy. Read more(https://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html).
More Information for Clinicians
Diagnostic assistance for malaria is available through DPDx.
How to Request
Clinicians who wish to obtain artesunate for severe malaria should contact the CDC Malaria Hotline at 770-488-7788 (M-F, 8am-4:30pm, Eastern time) or, after hours, the CDC Emergency Operations Center (EOC) at 770-488-7100, and request to speak with a CDC Malaria Branch clinician. A Malaria Branch clinician will provide clinical consultation by telephone and, if indicated, authorize the emergency release of artesunate from one of the CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
Requests for unapproved uses cannot be granted.
For non-emergency questions related to artesunate IV, contact the CDC Drug Service.
Benznidazole
Benznidazole is a 2-nitroimidazole trypanocidal agent that was introduced in 1971 for the treatment of Trypanosoma cruzi infection—i.e., Chagas disease, also known as American trypanosomiasis(https://www.cdc.gov/parasites/chagas/). Benznidazole is one of two drugs available from CDC for the treatment of Chagas disease (the other is nifurtimox). In the United States, the need to have drugs available for treating Chagas disease has been increasing, largely because of implementation of T. cruzi blood-donor screening in 2007, which has identified chronically infected persons (mainly Latin American immigrants) who might benefit from treatment and has heightened awareness of Chagas disease.
More Information for Clinicians
Diagnostic assistance for American trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of Chagas disease should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email chagas@cdc.gov) M-F 7:30am-4pm EST.
For emergencies (for example, acute Chagas disease with severe manifestations, Chagas disease in a newborn, or Chagas disease in an immunocompromised person) outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Botulism Antitoxin Heptavalent (Equine), Types A-G
Botulism Antitoxin Heptavalent (HBAT) contains equine-derived antibody to the seven known botulinum toxin types (A-G). HBAT is composed of <2% intact immunoglobulin G (IgG) and ≥90% Fab and F(ab’)2 immunoglobulin fragments. These fragments are created by the enzymatic cleavage and removal of Fc immunoglobulin components in a process sometimes referred to as despeciation. HBAT is supplied on an emergency basis for the treatment of persons thought to be suffering from botulism and works by neutralizing unbound toxin molecules. In 2010, HBAT became the only botulism antitoxin available in the United States for naturally occurring non-infant botulism.
It is available only from CDC because of its limited use and its relatively short expiration date. The antitoxin is stored at CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
BabyBIG® (botulism immune globulin) remains available for infant botulism through the California Infant Botulism Treatment and Prevention Program.
More Information for Clinicians
How to Request
Clinicians who suspect a diagnosis of botulism in a patient should immediately call their state health department’s 24-hour telephone number(https://www.cdc.gov/mmwr/international/relres.html) to maintain effective botulism surveillance and to facilitate rapid detection of outbreaks. The state health department will contact CDC to arrange for a clinical consultation by telephone and, if indicated, release of botulism antitoxin. State health departments requesting botulism antitoxin should contact the CDC Emergency Operations Center (EOC) at 770-488-7100. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5232a8.htm).
For non-emergency questions concerning botulism antitoxin, contact the CDC Drug Service.
Diethylcarbamazine (DEC)
DEC is an antihelminthic agent used for treatment of lymphatic filariasis (caused by infection with Wuchereria bancrofti, Brugia malayi, or Brugia timori), tropical pulmonary eosinophilia, and loiasis(https://www.cdc.gov/parasites/loiasis/); DEC also has prophylactic benefit for Loa loa infection. DEC has been used worldwide for more than 50 years. In the past, Wyeth-Ayerst Laboratories made DEC available as a licensed drug; in the late 1990s, because of unavailability of a bulk chemical supplier, Wyeth-Ayerst discontinued distribution of DEC in the United States.
More Information for Clinicians
Diagnostic assistance for filarial diseases is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of filarial diseases should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
After-hours emergencies: 1-770-488-7100
Diphtheria Antitoxin (Equine)
Diphtheria antitoxin (DAT) is used to prevent or treat diphtheria by neutralizing the toxins produced by Corynebacterium diphtheriae. DAT is a sterile, aqueous solution of the refined and concentrated proteins, chiefly globulins, containing antibodies obtained from the serum of horses that have been immunized against diphtheria toxin. DAT is available under an IND protocol sponsored by CDC and is released only for actual or suspected cases of diphtheria(https://www.cdc.gov/diphtheria/about/index.html). The antitoxin is stored at CDC Quarantine Stations located in major airports around the nation, ensuring delivery to any location in the United States within hours.
More Information for Clinicians
CDC’s Vaccine-Related Topics: Diphtheria Antitoxin(https://www.cdc.gov/diphtheria/dat.html)
How to Request
Clinicians who suspect a diagnosis of respiratory diphtheria can obtain DAT by contacting the Emergency Operations Center at 770-488-7100. They will be connected with the diphtheria duty officer, who will provide clinical consultation and, if indicated, initiate the release of diphtheria antitoxin.
For non-emergency questions concerning diphtheria antitoxin, contact the CDC Drug Service.
Eflornithine
Eflornithine is an antitrypanosomal agent that inhibits the enzyme ornithine decarboxylase. Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/); the choice of therapy depends on the infecting subspecies of the parasite and on the stage of the infection. Eflornithine is considered the drug of choice for the treatment of second-stage Trypanosoma brucei gambiense (West African) infection, with involvement of the central nervous system. It is not effective against T. b. rhodesiense (East African) infection (see melarsoprol). Although the manufacturer, Aventis, maintains its US licensure, eflornithine is not commercially available in the United States.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Melarsoprol
Melarsoprol is an organoarsenic compound with trypanocidal effects that has been used outside the United States since 1949. Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/); the choice of therapy depends on the infecting subspecies of the parasite and on the stage of the infection. Melarsoprol is used for the treatment of second-stage infection (involving the central nervous system). It is the only available therapy for second-stage Trypanosoma brucei rhodesiense (East African) infection, whereas eflornithine typically is used for second-stage T. b. gambiense (West African) infection.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Nifurtimox
Nifurtimox is a nitrofuran analog that was introduced in 1965 for the treatment of Trypanosoma cruzi infection—i.e., Chagas disease, also known as American trypanosomiasis(https://www.cdc.gov/parasites/chagas/). Nifurtimox is one of two drugs available from CDC for the treatment of Chagas disease (the other is benznidazole). In the United States, the need to have drugs available for treating Chagas disease has been increasing, largely because of implementation of T. cruzi blood-donor screening in 2007, which has identified chronically infected persons (mainly Latin American immigrants) who might benefit from treatment and has heightened awareness of Chagas disease.
More Information for Clinicians
Diagnostic assistance for American trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of Chagas disease should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email chagas@cdc.gov) M-F 7:30am-4pm EST.
For emergencies (for example, acute Chagas disease with severe manifestations, Chagas disease in a newborn, or Chagas disease in an immunocompromised person) outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Sodium Stibogluconate
Sodium stibogluconate (Pentostam®) is a pentavalent antimony compound used for treatment of leishmaniasis(https://www.cdc.gov/parasites/leishmaniasis). The three main clinical syndromes in humans are visceral, cutaneous, and mucosal leishmaniasis. Pentostam is a well-established antileishmanial agent that has been used in many countries of the world for more than half a century.
More Information for Clinicians
Diagnostic assistance for leishmaniasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of leishmaniasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
After-hours emergencies: 1-770-488-7100
Suramin
Suramin is a negatively charged, high-molecular-weight sulfated naphthylamine. It was introduced in the 1920s for the treatment of African trypanosomiasis (sleeping sickness)(https://www.cdc.gov/parasites/sleepingsickness/). Suramin generally is considered the drug of choice for first-stage Trypanosoma brucei rhodesiense (East African) infection, without involvement of the central nervous system. Pentamidine typically is used for first-stage T. b. gambiense (West African) infection.
More Information for Clinicians
Human African trypanosomiasis, WHO
Diagnostic assistance for African trypanosomiasis is available through DPDx.
How to Request
Contact the CDC Drug Service for more information.
Questions regarding treatment of African trypanosomiasis should be directed to CDC Parasitic Diseases Inquiries (404-718-4745; email parasites@cdc.gov) M-F 7:30am-4pm EST.
For emergencies outside of regular business hours, call the CDC Emergency Operations Center (770-488-7100) and ask for the person on call for Parasitic Diseases.
Vaccinia Vaccine, “Smallpox Vaccine”
Smallpox vaccine is made of live vaccinia virus derived from plaque purification cloning of Dryvax® (calf lymph vaccine, New York City Board of Health Strain) and grown in African Green Monkey kidney (Vero) cells and tested to be free of adventitious agents. It contains approximately 2.5 – 12.5 x 105 plaque-forming units per dose.
Smallpox was declared globally eradicated in 1980. In 1982, Wyeth Laboratories, the only active manufacturer of licensed vaccinia vaccine in the United States, discontinued production; and, in 1983, distribution to the civilian population was discontinued. Since January 1982, smallpox vaccination has not been required for international travelers, and International Certificates of Vaccination no longer include smallpox vaccination. ACAM2000® is a new-generation smallpox vaccine that was licensed in 2010 for use as a medical countermeasure held by the Strategic National Stockpile.
CDC recommends vaccinia vaccine for laboratory workers who directly handle a) cultures or b) animals contaminated or infected with nonhighly attenuated vaccinia virus, recombinant vaccinia viruses derived from nonhighly attenuated vaccinia strains, or other orthopoxviruses that infect humans (e.g., monkeypox, cowpox, vaccinia, and variola). Other health-care workers (e.g., physicians and nurses) whose contact with nonhighly attenuated vaccinia viruses is limited to contaminated materials (e.g., dressings) and who adhere to appropriate infection control measures are at lower risk for inadvertent infection than laboratory workers. However, because a theoretical risk for infection exists, vaccination can be offered to this group. Read more[PDF – 930KB](https://www.cdc.gov/mmwr/pdf/rr/rr5010.pdf).
More Information for Clinicians
Full Prescribing Information for ACAM2000®[PDF – 11 pages]
ACAM2000® Medication Guide[PDF – 6 pages]
CDC’s Vaccine-Related Topics: Smallpox Vaccine
How to Request
Smallpox vaccine must be administered by or under the supervision of the physician who registers with CDC.
Ancillary supplies, such as bifurcated needles (for administration) and 1 mL tuberculin syringes with 25 gauge x 5/8″ needles (for reconstitution), are supplied with the vaccine.
Contact the CDC Drug Service for more information.
Requests for unapproved uses cannot be granted.
Products No Longer Supplied by Drug Service*
Botulinum Toxoid
Pentavalent (ABCDE) botulinum toxoid is a combination of aluminum phosphate-adsorbed toxoid derived from formalin-inactivated types A, B, C, D, and E botulinum toxins, with formaldehyde and thimerosal used as preservatives. Botulinum toxoid was distributed by CDC under an IND protocol for at-risk persons who were actively working or expected to be working with cultures of Clostridium botulinum or the toxins; in 2011, CDC discontinued its program to supply this vaccine. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6042a3.htm).
Botulinum Antitoxin Types AB & E
In March 2010, CDC announced the availability of a new heptavalent botulinum antitoxin (HBAT, Cangene Corporation). HBAT replaced the licensed bivalent botulinum antitoxin AB and an investigational monovalent botulinum antitoxin E (BAT-AB and BAT-E, Sanofi Pasteur), becoming the only botulinum antitoxin available in the United States for naturally occurring non-infant botulism. Read more(https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5910a4.htm).
Vaccinia Immune Globulin (VIG)
Vaccinia immune globulin (VIG) is released from the CDC Strategic National Stockpile, if indicated, for the treatment of complications associated with vaccinia vaccination. Clinicians wishing to obtain VIG should contact the Emergency Operations Center (EOC) at 770-488-7100. They will be connected with CDC medical staff who can assist them in the diagnosis and management of patients with suspected complications of vaccinia vaccination.
*this list is not all-inclusive
Use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
USA: High-level isolation units in select Ebola hospitals report struggling to fund ongoing operations and sustain readiness.
Saturday, June 3rd, 2017Volume 23, Number 6—June 2017
Dispatch
Sustainability of High-Level Isolation Capabilities among US Ebola Treatment Centers
Main Article(https://wwwnc.cdc.gov/eid/article/23/6/17-0062_article)
Figure 1
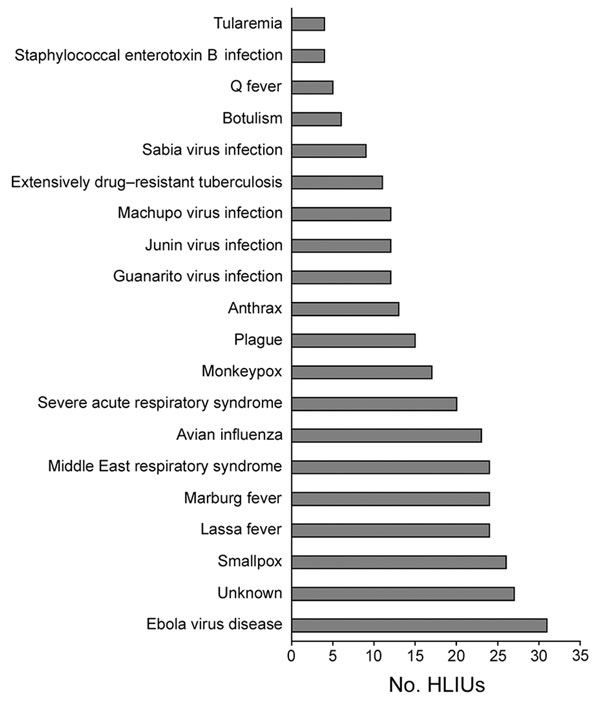
Figure 1. Diseases that 31 HLIUs reported they would treat, United States, 2016. HLIU, high-level isolation unit.
Main Article(https://wwwnc.cdc.gov/eid/article/23/6/17-0062_article)
Protecting the nation from deadly pathogens, man-made or natural: More difficult under the Prez’ budget.
Thursday, June 1st, 2017“….The Office of Public Health Preparedness and Response, which tracks outbreaks of disease, would be cut by $136 million, or 9.7 percent. The National Center for Emerging and Zoonotic Infectious Diseases — a branch of the Centers for Disease Control and Prevention that fights threats like anthrax and Ebola — would be cut by $65 million, or 11 percent.
The C.D.C.’s Center for Global Health would lose $76 million, or 18 percent. Its Emergency Operations Center, which conducts real-time monitoring of outbreak responses, and its Select Agents Program, which sets regulations in lethal toxin labs and helps researchers stay ahead of bioterrorists, face unspecified cuts as well…..”
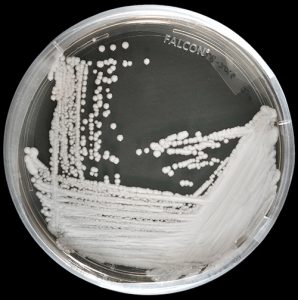
Candida auris: 61 cases of the fungus have been reported in the U.S. since 2013
Sunday, May 7th, 2017Candida auris is an emerging fungus that presents a serious global health threat. Healthcare facilities in several countries have reported that C. auris has caused severe illness in hospitalized patients. Some strains of Candida auris are resistant to all three major classes of antifungal drugs. This type of multidrug resistance has not been seen before in other species of Candida. Also of concern, C. auris can persist on surfaces in healthcare environments and spread between patients in healthcare facilities, unlike most other Candida species. CDC has developed Interim Recommendations(https://www.cdc.gov/fungal/diseases/candidiasis/recommendations.html) to help prevent the spread of C. auris.
C. auris is difficult to identify with standard laboratory methods and can be misidentified in labs without specific technology. CDC encourages all U.S. laboratory staff who identify C. auris strains to notify their state or local public health authorities and CDC at candidaauris@cdc.gov. Find answers to frequently asked questions about C. auris on our questions and answers page(https://www.cdc.gov/fungal/diseases/candidiasis/candida-auris-qanda.html) and in the Candida auris: Interim Recommendations(https://www.cdc.gov/fungal/diseases/candidiasis/recommendations.html).
CDC is working with state and local health departments to identify and investigate cases of C. auris. The following map displays where C. auris cases have been identified in the United States as of April 13, 2017.
Data Table

Trump & Deadly Disease
Saturday, March 25th, 2017- “…..President Trump’s budget would cut funding for the National Institutes of Health by 18 percent.
- It would cut the State Department and the United States Agency for International Development, a key vehicle for preventing and responding to outbreaks before they reach our shores, by 28 percent.
- And the repeal of the Affordable Care Act would kill the billion-dollar Prevention and Public Health Fund, which provides funding for the Centers for Disease Control and Prevention to fight outbreaks of infectious disease.
- (While the budget also calls for the creation of an emergency fund to respond to outbreaks, there is no indication that it would offset the other cuts, or where the money would come from.)
- We are already witnessing an outbreak of influenza in birds — the H7N9 strain, in China — that could be the source for the next human pandemic. Since October, over 500 people have been infected; more than 34 percent have died. Most victims had contact with infected poultry, yet three recent clusters appear to be from person-to-person transmission. Will H7N9 mutate to become easily transmitted between humans? We don’t know. But without sufficient supplies of a vaccine, we are not prepared to stop it…….”
CDC’s PHPR (Office of Public Health Preparedness and Response) In Action
Friday, March 17th, 2017What We Do

An emergency can happen at any moment, and every community in the U.S. must be ready to respond. A pandemic, natural disaster, or chemical or radiological release often strikes without warning. The costs—both economic and human—can be dear.
-

READY FOR EMERGENCIES
In an emergency, you can’t respond effectively if you’re not ready
-

EMERGENCY OPERATIONS
Bringing resources and experts together to respond to emergencies quickly and to scale
-

CRITICAL MEDICINES
AND SUPPLIESMaking sure critical medicines and supplies can get to the right place at the right time
-

LABORATORY RESPONSE
Building capacity to quickly detect, diagnose, and treat those who are impacted by health emergencies
New quarantine rules: CDC trying to out-Trump Trump. And that’s making people jumpy…..
Thursday, February 2nd, 2017“….Under the old rules, the CDC’s authority was primarily limited to detaining people entering the country or crossing state lines. The agency was also limited to quarantining people who had one of about a dozen diseases, including cholera, diphtheria, tuberculosis, plague, smallpox and yellow fever.
Yet even then, the CDC rarely exercised these powers and generally deferred to state and local health officials.
With the new rules, the CDC would be able to detain people anywhere in the country without getting approval from state and local officials.
The agency could also apprehend people to assess their health if they are exhibiting medical problems such as a high fever, headache, cramps and other symptoms that could be indicative of a dangerous infectious disease…..”