Archive for the ‘CDC’ Category
CDC Recommendations for Subsequent Zika IgM Antibody Testing
Thursday, June 23rd, 2016This is an official
CDC HEALTH UPDATE
Distributed via the CDC Health Alert Network
June 21, 2016, 1140 EDT (11:40 AM EDT)
CDCHAN-00392
CDC Recommendations for Subsequent Zika IgM Antibody Testing
Summary
Testing for Zika virus infection using real-time reverse-transcription polymerase chain reaction (rRT-PCR)
molecular assays is now commercially available. When requesting Zika rRT-PCR testing from a
commercial laboratory, providers should be aware that commercial laboratories performing rRT-PCR
currently do not also offer Zika IgM enzyme-linked immunosorbent assay (ELISA) or confirmatory
serologic testing (plaque reduction neutralization test, or PRNT). Therefore, if possible, providers should
store a serum aliquot for subsequent Zika IgM ELISA testing if the rRT-PCR assay is negative. Otherwise,
collection of an additional serum sample may be necessary.
Recommendations
• rRT-PCR (molecular) testing should be performed for patients possibly exposed to Zika virus who
have symptoms consistent with Zika virus infection
• Providers who request molecular testing for Zika virus infection from a commercial testing
laboratory are advised to retain and store in a refrigerator (2-8°C) an aliquot of the patient’s
serum for subsequent Zika IgM ELISA testing if the rRT-PCR is negative
• For specimens that are rRT-PCR negative from the commercial laboratory and no stored serum
specimen is available, another serum specimen should be collected within 12 weeks of symptom
onset for Zika IgM ELISA testing
• Appropriate samples for molecular testing are serum samples collected <7 days and urine
samples collected <14 days after symptom onset. Urine should always be collected with a
patient-matched serum specimen.
Background
Molecular assays for detection of Zika virus RNA are now commercially available under Emergency Use
Authorizations (EUAs) issued by the Food and Drug Administration (FDA). CDC recommends molecular
testing using rRT-PCR for serum samples collected <7 days and urine samples collected <14 days after
symptom onset. A positive rRT-PCR test is confirmation of Zika virus infection. However, because of the
decline in the level of viremia over time and possible inaccuracy in reporting of dates of illness onset, a
negative rRT-PCR result does not exclude Zika virus infection. In such cases, CDC recommends
serologic testing by ELISA for Zika IgM antibody.
Currently, commercial laboratories that offer rRT-PCR testing do not provide Zika IgM ELISA testing with
PRNT confirmation and have no routine process to forward specimens to another testing laboratory.
Therefore, when requesting Zika rRT-PCR testing from a commercial laboratory, providers should retain
an aliquot of the serum for Zika IgM ELISA testing if the rRT-PCR testing is negative. Blood should be
collected and processed per routine guidelines (collected in a serum separator tube with serum aliquots
transferred to new vials), and one of the serum aliquots should be stored in a refrigerator (2-8°C) until it is
known if additional IgM testing is indicated. If a serum aliquot cannot be stored or is not available, but
further testing is indicated, a new blood sample should be collected. Serum samples for IgM testing
should be collected from patients within 12 weeks of symptom onset. Providers should contact their local
health department to discuss IgM testing of stored or newly collected serum from patients who are rRTPCR
negative.
For More Information
• Zika virus specimen collection:
http://www.cdc.gov/zika/hc-providers/body-fluids-collection-submission.html.
• Interim guidance for Zika virus testing of urine:
http://www.cdc.gov/mmwr/volumes/65/wr/mm6518e1.htm
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and
controlling diseases and injuries; enhances health decisions by providing credible information on critical health
issues; and promotes healthy living through strong partnerships with local, national, and international
organizations.
____________________________________________________________________________________
Categories of Health Alert Network messages:
Health Alert Requires immediate action or attention; highest level of importance
Health Advisory May not require immediate action; provides important information for a specific incident or situation
Health Update Unlikely to require immediate action; provides updated information regarding an incident or situation
HAN Info Service Does not require immediate action; provides general public health information
##This message was distributed to state and local health officers, state and local epidemiologists, state
and local laboratory directors, public information officers, epidemiologists, HAN coordinators, and clinician
organizations##
CDC’s Interim Zika Response Plan
Wednesday, June 15th, 2016The 57-page plan: http://files.ctctcdn.com/6f0559fe101/01ee9685-9672-4d5c-88e5-0873b30c950f.pdf
CDC will support and help states with key tasks at different stages of the outbreak
Phase level 0 —signifying preparedness activity for when the vector is present or possible in the state—to level 4, when widespread local Zika infections are occurring is several jurisdictions within a state. Most states are currently in phase 0 or 1, meaning Aedes mosquitoes are biting and travel-related or sexually transmitted cases have occurred.
Phase 2 would be a single locally acquired case or a case cluster in a single household.
Phase 3 consists of widespread local transmission contained to a 1-mile area
Phase 4 is widespread transmission in multiple locations.
Seven Multistate Outbreaks of Human Salmonella Infections Linked to Live Poultry in Backyard Flocks (n=324)
Sunday, June 5th, 2016The Strategic National Stockpile’s Unique Role in Zika Prevention
Wednesday, May 11th, 2016
The first thing that comes to mind when people think about the Strategic National Stockpile (SNS) is probably a big warehouse with lots of medicines and supplies. What many do not know is that even when the SNS does not have the specific medicines or supplies needed to combat a public health threat, SNS experts can play a key role in working with medical supply chain partners to locate and purchase products during an emergency response.
The involvement of the SNS in the Zika virus response is a perfect example of this little-known, but significant, role. Zika is spread to people primarily through the bite of an Aedes aegypti mosquito infected with Zika virus, although Aedes albopictus mosquitoes may also spread the virus. Recent outbreaks of Zika in the Americas, Caribbean, and Pacific Islands have coincided with increased reports of microcephaly and other birth defects as well as Guillain-Barré syndrome. As a result, the Centers for Disease Control and Prevention’s (CDC) response is focused on limiting the spread of Zika virus. Prevention is key for Zika control, because there is no vaccine or medicine for Zika virus. This is where the SNS comes in.
Controlling mosquito populations is key to prevention
During a public health emergency, CDC can deploy the SNS for medicines and supplies or can use SNS’ contracting abilities to access materials and services that can be used to prevent or treat diseases that threaten U.S. health security. Controlling the mosquito population and addressing other known routes of infection are important to limit the spread of Zika virus in U.S. territories. The SNS is providing immediate vector control services and preventive supplies for pregnant women to protect themselves from mosquito bites. Pregnant women are particularly vulnerable because they can pass Zika virus to their fetuses, which can cause microcephaly and other brain defects.
Before the Zika virus outbreak, the SNS did not stock or purchase medicines or supplies to respond to illnesses spread by mosquitoes, ticks, and other insects. In response to this outbreak, SNS staff are working with CDC procurement experts to award and implement immediate, short-term contracts to deploy materials and services to control the mosquito populations responsible for Zika transmission. These contracts allow CDC to work with territorial public health jurisdictions to treat areas where mosquitoes breed and live, as well as areas where pregnant women live.
Zika Prevention Kits help pregnant women protect themselves
 The SNS is creating Zika Prevention Kits for pregnant women in U.S. territories. These kits are being distributed as an effort to help prevent Zika infection in pregnant women and to reduce the number of babies born with birth defects caused by Zika, such as microcephaly and other brain defects. Through donations from the CDC Foundation and its partners and by purchasing products, the SNS has obtained materials for the kits – including insect repellent, larvicides, mosquito netting, condoms to prevent sexual transmission of Zika, and educational materials. The SNS is rapidly assembling these materials in reusable bags that can be given to pregnant women.
The SNS is creating Zika Prevention Kits for pregnant women in U.S. territories. These kits are being distributed as an effort to help prevent Zika infection in pregnant women and to reduce the number of babies born with birth defects caused by Zika, such as microcephaly and other brain defects. Through donations from the CDC Foundation and its partners and by purchasing products, the SNS has obtained materials for the kits – including insect repellent, larvicides, mosquito netting, condoms to prevent sexual transmission of Zika, and educational materials. The SNS is rapidly assembling these materials in reusable bags that can be given to pregnant women.
The SNS has sent nearly 7,000 kits to affected areas, and more are planned. Each U.S. territory is identifying the best way to get the kits to pregnant women. In Puerto Rico, local public health officials have partnered with clinics that are part of the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) so they can reach expectant mothers. WIC already interacts with this population through its healthcare and nutritional services for low-income women, infants, and children. Local obstetrician offices are also being used to distribute these kits.
In the past, the SNS primarily focused on warehousing products and deploying those products for public health threats related to bioterrorism, pandemics, and natural disasters. With every emergency response, it has become more evident that the SNS can play a much larger role, especially when specialty products, products in high demand, and medical countermeasures are needed to secure the nation’s health. As one of the federal government’s leading groups of medical supply chain and logistics experts, the SNS at CDC has the ability to coordinate with industry partners to rapidly procure and transport medicines and supplies and serve specific populations in a public health emergency.
Posted on May 9, 2016 by
National Day of Action: Baby steps you can take to be better prepared at home, in your community, and on the go.
Monday, May 2nd, 2016
To mark the National Day of Action, there are hundreds of little steps you can take to be better prepared at home, in your community, and on the go. Here are a few quick action steps you can take today!
- Sign up for local alerts and warnings. There are different types of alerts and warnings that you can receive about weather conditions and other emergency situations. Check with your local health department or emergency management agency to see how they share emergency information, whether it is through emergency texts, phone calls, digital road signs, social media, or sirens. You can even download an emergency app from FEMA, The Red Cross, or theWeather Channel.
- Create and test communication plans. Have a discussion with your family before a disaster strikes and make a plan for how you will connect to each other.
- Complete a contact card for every member of your family. Make sure to keep these cards with you at all times
- Choose an emergency contact. Keep in mind that it might be easier to reach a friend or relative who lives out of town.
- Identify a meeting place in your neighborhood and your city or town where your family could gather if there is an emergency.

- Build an emergency supply kit. Make sure you have at least a three day supply of food and water for each person in your family. Also include health supplies, personal care items, safety supplies, electronics, and copies of important documents.
- Safeguard documents. Identify financial and legal documents, medical information, household identification, and key contact information you might need after a disaster. Use this helpful checklist to take an inventory and not forget to safeguard any critical documents.
- Document and insure property. Different types of insurance cover different types of damage after a disaster. Make sure you understand your insurance policies and minimize potential losses.
- Make your property safer. Make property improvements to reduce damage to your property during a disaster and prevent potential injuries from different types of emergencies.
- Conduct a drill. Practice emergency response actions for disasters that might happen in your community.
- Conduct an exercise of a disaster scenario. Use mock scenarios for different types of disasters to review and improve your emergency plan. You might consider participating in a community-wide tabletop exercise for different emergency situations. In your home, you can practice a fire drill, tornado drill, or earthquake drill.
- Plan with neighbors. Many people rely on their neighbors after a disaster. Make sure you start the conversation about preparedness before a disaster strikes. Know the needs of your neighbors and be ready to help in an emergency.
- Participate in a class, training or discussion. Contact your local emergency management agency to see what trainings are available in your community, or consider enrolling in a first aid or CPR course at your local Red Cross.
** In disasters and pandemics, kids aren’t just little adults
Monday, April 25th, 2016How are children different from adults?
Disasters affect children differently than they do adults. Learn more about the unique needs of children during and after disasters.

- Children’s bodies are different from adults’ bodies.
- They are more likely to get sick or severely injured.
- They breathe in more air per pound of body weight than adults do.
- They have thinner skin, and more of it per pound of body weight (higher surface-to-mass ratio).
- Fluid loss (e.g. dehydration, blood loss) can have a bigger effect on children because they have less fluid in their bodies.
- They are more likely to lose too much body heat.
- They spend more time outside and on the ground. They also put their hands in their mouths more often than adults do.
- They are more likely to get sick or severely injured.
- Children need help from adults in an emergency.
- They don’t fully understand how to keep themselves safe.
- Older children and adolescents may take their cues from others.
- Young children may freeze, cry, or scream.
- They may not be able to explain what hurts or bothers them.
- They are more likely to get the care they need when they have parents or other caregivers around.
- Laws require an adult to make medical decisions for a child.
- There is limited information on the ways some illnesses and medicines affect children. Sometimes adults will have to make decisions with the information they have.
- They don’t fully understand how to keep themselves safe.
- Mental stress from a disaster can be harder on children.
- They feel less of a sense of control.
- They understand less about the situation.
- They have fewer experiences bouncing back from hard situations.
Microcephaly and Other Birth Defects & Zika: Cause and Effect
Thursday, April 14th, 2016CDC Concludes Zika Causes Microcephaly and Other Birth Defects
Media Statement
For Immediate Release: Wednesday, April 13, 2016
Contact: Media Relations,
(404) 639-3286
Scientists at the Centers for Disease Control and Prevention (CDC) have concluded, after careful review of existing evidence, that Zika virus is a cause of microcephaly and other severe fetal brain defects. In the report published in the New England Journal of Medicine, the CDC authors describe a rigorous weighing of evidence using established scientific criteria.
“This study marks a turning point in the Zika outbreak. It is now clear that the virus causes microcephaly. We are also launching further studies to determine whether children who have microcephaly born to mothers infected by the Zika virus is the tip of the iceberg of what we could see in damaging effects on the brain and other developmental problems,” said Tom Frieden, M.D., M.P.H., director of the CDC. “We’ve now confirmed what mounting evidence has suggested, affirming our early guidance to pregnant women and their partners to take steps to avoid Zika infection and to health care professionals who are talking to patients every day. We are working to do everything possible to protect the American public.”
Background
The report notes that no single piece of evidence provides conclusive proof that Zika virus infection is a cause of microcephaly and other fetal brain defects. Rather, increasing evidence from a number of recently published studies and a careful evaluation using established scientific criteria supports the authors’ conclusions.
The finding that Zika virus infection can cause microcephaly and other severe fetal brain defects means that a woman who is infected with Zika during pregnancy has an increased risk of having a baby with these health problems. It does not mean, however, that all women who have Zika virus infection during pregnancy will have babies with problems. As has been seen during the current Zika outbreak, some infected women have delivered babies that appear to be healthy.
Establishing this causal relationship between Zika and fetal brain defects is an important step in driving additional prevention efforts, focusing research activities, and reinforcing the need for direct communication about the risks of Zika. While one important question about causality has been answered, many questions remain. Answering these will be the focus of ongoing research to help improve prevention efforts, which ultimately may help reduce the effects of Zika virus infection during pregnancy.
At this time, CDC is not changing its current guidance as a result of this finding. Pregnant women should continue to avoid travel to areas where Zika is actively spreading. If a pregnant woman travels to or lives in an area with active Zika virus transmission, she should talk with her healthcare provider and strictly follow steps to prevent mosquito bites and to prevent sexual transmission of Zika virus. We also continue to encourage women and their partners in areas with active Zika transmission to engage in pregnancy planning and counseling with their health care providers so that they know the risks and the ways to mitigate them.
How to protect your kids in a disaster when you’re at work and they’re at school?
Sunday, March 13th, 2016
http://www.cdc.gov/phpr/readywrigley/documents/backpack_emergency_card.pdf
CDC: Caring for Infants and Children with Possible Zika Virus Infection
Tuesday, March 1st, 2016Update: Interim Guidelines for Health Care Providers Caring for Infants and Children with Possible Zika Virus Infection — United States, February 2016
Early Release / February 19, 2016 / 65(7);1–6
Katherine E. Fleming-Dutra, MD1; Jennifer M. Nelson, MD2,3; Marc Fischer, MD4; J. Erin Staples, MD, PhD4; Mateusz P. Karwowski, MD2,5; Paul Mead, MD4; Julie Villanueva, PhD6; Christina M. Renquist, MPH7; Anna A. Minta, MD2,8; Denise J. Jamieson, MD9; Margaret A. Honein, PhD7; Cynthia A. Moore, MD, PhD7; Sonja A. Rasmussen, MD10 (View author affiliations)
CDC has updated its interim guidelines for U.S. health care providers caring for infants born to mothers who traveled to or resided in areas with Zika virus transmission during pregnancy and expanded guidelines to include infants and children with possible acute Zika virus disease (1). This update contains a new recommendation for routine care for infants born to mothers who traveled to or resided in areas with Zika virus transmission during pregnancy but did not receive Zika virus testing, when the infant has a normal head circumference, normal prenatal and postnatal ultrasounds (if performed), and normal physical examination. Acute Zika virus disease should be suspected in an infant or child aged <18 years who 1) traveled to or resided in an affected area within the past 2 weeks and 2) has ≥2 of the following manifestations: fever, rash, conjunctivitis, or arthralgia. Because maternal-infant transmission of Zika virus during delivery is possible, acute Zika virus disease should also be suspected in an infant during the first 2 weeks of life 1) whose mother traveled to or resided in an affected area within 2 weeks of delivery and 2) who has ≥2 of the following manifestations: fever, rash, conjunctivitis, or arthralgia. Evidence suggests that Zika virus illness in children is usually mild (2). As an arboviral disease, Zika virus disease is nationally notifiable. Health care providers should report suspected cases of Zika virus disease to their local, state, or territorial health departments to arrange testing and so that action can be taken to reduce the risk for local Zika virus transmission. As new information becomes available, these guidelines will be updated: http://www.cdc.gov/zika/.
Zika virus is primarily transmitted to humans through the bite of Aedes species mosquitoes, most commonly Aedes aegypti and possibly Aedes albopictus (3). Zika virus was first detected in the Region of the Americas (Americas) in Brazil in the spring of 2015 (4) and had spread to 26 countries and territories in the Americas as of February 17, 2016 (http://www.cdc.gov/zika/geo/active-countries.html). In October 2015, a marked increase in the number of infants with microcephaly was reported in Brazil (5). Because of the temporal and geographic occurrence of Zika virus infection in pregnant women before the reported increase in microcephaly, a possible association with prenatal Zika virus infection was postulated (5). Laboratory evidence from a limited number of cases with microcephaly has supported this potential association (6,7). Other documented modes of Zika virus transmission include intrapartum transmission from a mother with viremia to her infant, sexual transmission, and laboratory exposures (8–11). Additionally, blood transfusion (10) and organ or tissue transplantation pose theoretical risks for transmission. There is no reported evidence of transmission through breastfeeding, although Zika virus RNA has been found in breast milk (9).
Although the exact incubation period of Zika virus disease has yet to be determined, evidence from case reports and experience from related flavivirus infections indicate that the incubation period likely is 3 days to 2 weeks (12). Symptomatic disease is generally mild and characterized by two or more of the following: acute onset of fever, rash, arthralgia, or nonpurulent conjunctivitis (2,13). The rash associated with Zika virus disease has been described as pruritic (13) and maculopapular (14).
The spectrum of Zika virus disease in neonates infected in the perinatal period is unknown. Perinatal transmission of Zika virus infection to infants from mothers infected near the time of delivery has been reported in two cases; one of these infants was asymptomatic, and the other had thrombocytopenia and a diffuse rash (9). Mother-to-infant transmission of dengue virus, a related flavivirus, during the perinatal period has resulted in findings in the newborn ranging from no symptoms to severe illness (including fever, thrombocytopenia, and hemorrhage), most often with fever onset during the first week of life (15). Similarly West Nile virus, another mosquito-borne flavivirus, has been transmitted during the perinatal period from three mothers to their infants, with each infant having one of the following manifestations: rash, viral encephalitis, and viral meningitis (16). The clinical features that might be observed in infants who acquire Zika virus during the perinatal period are currently unknown.
Available evidence regarding the spectrum of Zika virus disease in infants and children who are infected through mosquito bites indicates that most children are asymptomatic or have mild illness, similar to the findings seen in adults infected with Zika virus disease. In the outbreak in Yap Island, Micronesia, in 2007, among persons with clinical illness (age range = 1–76 years), fever, macular or papular rash, arthralgia, and conjunctivitis were the most common signs and symptoms (2). In that outbreak, children aged 0–19 years had lower attack rates of confirmed and probable Zika virus disease than did adults aged 20–59 years (2). Additional published data are available for 10 children, aged 3–16 years (17–22) with Zika virus disease in Africa, Asia, South America, and the Pacific. All 10 children had fever, but none had rash, two had conjunctivitis, and three had arthralgia. Vomiting was reported in two children (17,22), and diarrhea was reported in two children (22). Among eight recent travel-related cases among children in the United States, all had rash and at least one other sign or symptom (fever, arthralgia, nonpurulent conjunctivitis) (CDC, unpublished data, 2016).
Deaths from Zika virus infection appear to be rare in persons of all ages. One death was reported in a female aged 15 years with sickle cell disease (hemoglobin SC), who experienced 4 days of fever, myalgia, abdominal pain and jaundice (18). A blood sample collected 5 days after illness onset was positive by reverse transcription–polymerase chain reaction (RT-PCR) for Zika virus RNA and negative for dengue, chikungunya, and yellow fever viruses (18). This patient died from complications of sickle cell disease after developing severe acute respiratory distress syndrome, hemothorax, and splenic sequestration (18). An additional death was reported in a female aged 16 years whose symptoms included headache, nausea, and petechiae; blood samples obtained 7 days after illness onset were positive by RT-PCR for Zika virus RNA (23). No further information was reported (23).
Guillain-Barré syndrome has been reported following Zika virus infection, although a causal link has not been established. Overall Guillain-Barré syndrome incidence appears to increase with increasing age (24). However, it is unclear how often Guillian-Barré syndrome after Zika virus infection has occurred in children (10). In French Polynesia, among 38 reported cases of Guillian-Barré syndrome after Zika virus infection, none occurred among children (25). One report from Brazil refers to six patients, aged 2–57 years, with neurologic syndromes (four with Guillain-Barré and two with acute disseminated encephalomyelitis) after laboratory-confirmed Zika virus infection; however, no further data were reported (13).
Updated Recommendations for the Evaluation and Testing of Infants with Possible Congenital Zika Virus Infection
Congenital infections result from intrauterine transmission from mother to fetus during pregnancy. Testing of infants with possible congenital Zika virus infection who were born to mothers who traveled to or resided in areas affected by Zika virus during pregnancy should be guided by 1) whether the infant had microcephaly or intracranial calcifications detected prenatally or at birth and 2) the mother’s Zika virus testing results. The results of previous prenatal ultrasounds and maternal Zika virus testing should be reviewed, and a thorough newborn physical examination, with assessment of head (occipitofrontal) circumference, length, and weight, should be performed (26,27). The evaluation of infants with microcephaly or intracranial calcifications or infants whose mothers have positive or inconclusive test results for Zika virus infection remains the same as described in the recommendations released on January 26 (Figure) (Box 1,2,3) (1). Infants without microcephaly or intracranial calcifications whose mothers have negative Zika virus test results or who were not tested for Zika virus should receive routine care (Figure). Because information on the effects of congenital Zika virus infection is limited, health care providers should exercise clinical judgment in the assessment of newborns with abnormalities other than microcephaly or intracranial calcifications who were born to mothers who traveled to or resided in an area with active Zika virus transmission during pregnancy. For these infants, health care providers should consider testing the mother before testing the infant. These guidelines will be updated as additional information becomes available.
Guidelines for Evaluation and Management of Infants and Children Aged <18 Years with Possible Acute Zika Virus Disease
Acute Zika virus disease should be suspected in an infant or child aged <18 years who 1) traveled to or resided in an affected area within the past 2 weeks and 2) has two or more of the following manifestations: fever, rash, conjunctivitis, or arthralgia. Acute Zika virus disease should also be suspected in an infant in the first 2 weeks of life 1) whose mother traveled to or resided in an affected area within 2 weeks of delivery and 2) who has two or more of the following manifestations: fever, rash, conjunctivitis, or arthralgia. Arthralgia can be difficult to detect in infants and young children and can manifest as irritability, walking with a limp (for ambulatory children), difficulty moving or refusing to move an extremity, pain on palpation, or pain with active or passive movement of the affected joint. Infants and older children can acquire Zika virus through mosquito-borne transmission. Infants can also be infected perinatally if the mother became infected with Zika virus during travel to or residence in an area with Zika virus transmission within 2 weeks of delivery. Infants whose mothers reported illness consistent with Zika virus disease near the time of delivery should be monitored for signs and symptoms of Zika virus disease. If an infant shows signs and symptoms of acute Zika virus disease within the first 2 weeks of life, both the mother and infant should be tested for Zika virus infection. Persons might be exposed to Zika virus infection through sexual contact with a person who has traveled to or resided in an area affected by Zika virus (11).
Evaluation of infants and children for acute (symptom onset within the past 7 days) Zika virus infection should include testing of serum and, if obtained for other reasons, cerebrospinal fluid (CSF) specimens for evidence of Zika virus RNA using RT-PCR. If Zika virus RNA is not detected and symptoms have been present for ≥4 days, serum may be tested for Zika virus immunoglobulin M (IgM) and neutralizing antibodies, and dengue virus IgM and neutralizing antibodies (Box 1). Laboratory evidence of Zika virus infection in an infant or child would include, in any clinical specimen, detectable Zika virus in culture, Zika virus RNA or antigen, or a clinical specimen positive for Zika virus IgM with confirmatory neutralizing antibody titers ≥4-fold higher than dengue virus neutralizing antibody titers (1). If Zika virus antibody titers are <4-fold higher than dengue virus neutralizing antibody titers, test results for Zika virus are considered inconclusive (1). More information on laboratory testing can be found at http://www.cdc.gov/zika/state-labs/index.html. Health care providers should notify their local, state or territorial health department of suspected Zika cases to arrange testing and so that action can be taken to decrease the risk for local transmission in areas with Aedes species mosquitoes.
Illness associated with Zika virus is usually mild in children, and treatment of Zika virus infection involves supportive care. Nonsteroidal anti-inflammatory drugs (NSAIDs) should be avoided until dengue virus is ruled out as the cause of illness, because of the potential for hemorrhagic complications of dengue fever, and should be avoided in all children aged <6 months (28,29). Aspirin should not be used in children with acute viral illnesses because of its association with Reye’s syndrome (30). The decision to obtain additional laboratory tests, diagnostic studies, and infectious disease consultation should be based on clinical judgment as guided by findings from a complete history and physical examination. Information on long-term outcomes among infants and children with acute Zika virus disease is limited (10); until more evidence is available to inform recommendations, routine pediatric care is advised for these infants and children.
Guidelines for Breastfeeding for Mothers with Zika Virus Infection
Zika virus RNA has been identified in breast milk, but attempts to culture the virus have been unsuccessful (9). No cases of Zika virus infection associated with breastfeeding have been reported. CDC encourages mothers with Zika virus infection and living in areas with ongoing Zika virus transmission to breastfeed their infants. Current evidence suggests that the benefits of breastfeeding outweigh the theoretical risks of Zika virus transmission through breast milk.
Prevention of Zika Virus Infection in Infants and Children
Prevention of mosquito bites is the primary means of preventing Zika virus infection in persons of all ages traveling to or residing in areas with local Zika virus transmission. Mosquito bite prevention includes using air conditioning or window and door screens when indoors, wearing long-sleeved shirts and long pants, using permethrin-treated clothing and gear, and using insect repellents. When used as directed on the product label, most Environmental Protection Agency–registered insect repellents can be used to protect children aged ≥2 months against mosquito bites. Oil of lemon eucalyptus should not be used in children aged <3 years (http://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/protection-against-mosquitoes-ticks-other-arthropods). Mosquito netting can be used to cover infants in carriers, strollers, or cribs to protect them from mosquito bites. Information on the safe use of insect repellents in children is available at http://www.epa.gov/insect-repellents/using-insect-repellents-safely-and-effectively.
Persons with Zika virus infection should take steps to prevent mosquito bites for at least the first week of illness to decrease the risk for human-to-mosquito-to-human transmission. Health care providers should educate parents and caregivers about mosquito bite prevention in infants and children if they are traveling to or residing in areas affected by Zika virus; mosquitoes also carry other viruses in addition to Zika. More information about prevention of Zika virus infection can be found at http://www.cdc.gov/zika/prevention/index.html.
References
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7. CrossRef PubMed
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360:2536–43. CrossRef PubMed
- Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014;44:302–7. CrossRef PubMed
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas— Region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep 2016;65:55–8. CrossRef PubMed
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. ; Brazilian Medical Genetics Society–Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65:59–62. CrossRef PubMed
- Martines RB, Bhatnagar J, Keating MK, et al. Notes from the field: Evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil. MMWR Morb Mortal Wkly Rep 2016;65:159–60. CrossRef PubMed
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med 2016; Epub ahead of print. CrossRef
- The Subcommittee on Arbovirus Laboratory Safety of the American Committee on Arthropod-Borne Viruses. Laboratory safety for arboviruses and certain other viruses of vertebrates. Am J Trop Med Hyg 1980;29:1359–81. PubMed
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014;19:20751. CrossRef PubMed
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barre syndrome. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2015.
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:120–1. CrossRef PubMed
- Rudolph KE, Lessler J, Moloney RM, Kmush B, Cummings DA. Incubation periods of mosquito-borne viral infections: a systematic review. Am J Trop Med Hyg 2014;90:882–91. CrossRef PubMed
- Minstério de Saúde. Protocolo de vigilância e resposta à ocorrência de microcefalia relacionada à infecção pelo vírus Zika 2015. http://portalsaude.saude.gov.br/images/pdf/2015/dezembro/09/Microcefalia—Protocolo-de-vigil–ncia-e-resposta—vers–o-1—-09dez2015-8h.pdf
- Kwong JC, Druce JD, Leder K. Zika virus infection acquired during brief travel to Indonesia. Am J Trop Med Hyg 2013;89:516–7. CrossRef PubMed
- Pouliot SH, Xiong X, Harville E, et al. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv 2010;65:107–18. PubMed
- O’Leary DR, Kuhn S, Kniss KL, et al. Birth outcomes following West Nile Virus infection of pregnant women in the United States: 2003-2004. Pediatrics 2006;117:e537–45. CrossRef PubMed
- Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015;21:722–4. CrossRef PubMed
- Arzuza-Ortega L, Pérez-Tatis G, López-García H, et al. Fatal Zika virus infection in girl with sickle cell disease, Colombia[Letter]. Emerg Infect Dis 2016. Epub ahead of print. CrossRef
- Dupont-Rouzeyrol M, O’Connor O, Calvez E, et al. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis 2015;21:381–2. CrossRef PubMed
- Heang V, Yasuda CY, Sovann L, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012;18:349–51. CrossRef PubMed
- MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954;48:139–45. CrossRef PubMed
- Olson JG, Ksiazek TG, Suhandiman , Triwibowo . Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981;75:389–93. CrossRef PubMed
- Centro de operaçõs de emergênecias em saúde pública sobre microcefalias. Informe epidemiológico no 02/2015—Semana epidemiológica 47 (22 a 28/11/2015): Monitoramento dos casos de microcefalias no Brasil. http://portalsaude.saude.gov.br/images/pdf/2015/novembro/30/COES-Microcefalias—Informe-Epidemiol–gico—SE-47—30nov2015.pdf
- Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology 2011;36:123–33. CrossRef PubMed
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus infection outbreak, French Polynesia. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2014.
- World Health Organization. The WHO child growth standards. Geneva, Switzerland: World Health Organization. http://www.who.int/childgrowth/standards/en/
- University of Calgary. Welcome to the Fenton Preterm Growth Chart: 2013 Growth Chart. http://ucalgary.ca/fenton/2013chart
- Sullivan JE, Farrar HC; Section on Clinical Pharmacology and Therapeutics; Committee on Drugs. Fever and antipyretic use in children. Pediatrics 2011;127:580–7. CrossRef PubMed
- Tomashek KM, Sharp TM, Margolis HS. Dengue. Chapter 3. In: Brunette GW, ed. CDC Health information for international travel 2016.
- Hurwitz ES, Barrett MJ, Bregman D, et al. Public Health Service study of Reye’s syndrome and medications. Report of the main study. JAMA 1987;257:1905–11. CrossRef PubMed
 FIGURE. Interim guidelines for the evaluation and testing of infants whose mothers traveled to or resided in an area with ongoing Zika virus transmission* during pregnancy†,§,¶
FIGURE. Interim guidelines for the evaluation and testing of infants whose mothers traveled to or resided in an area with ongoing Zika virus transmission* during pregnancy†,§,¶
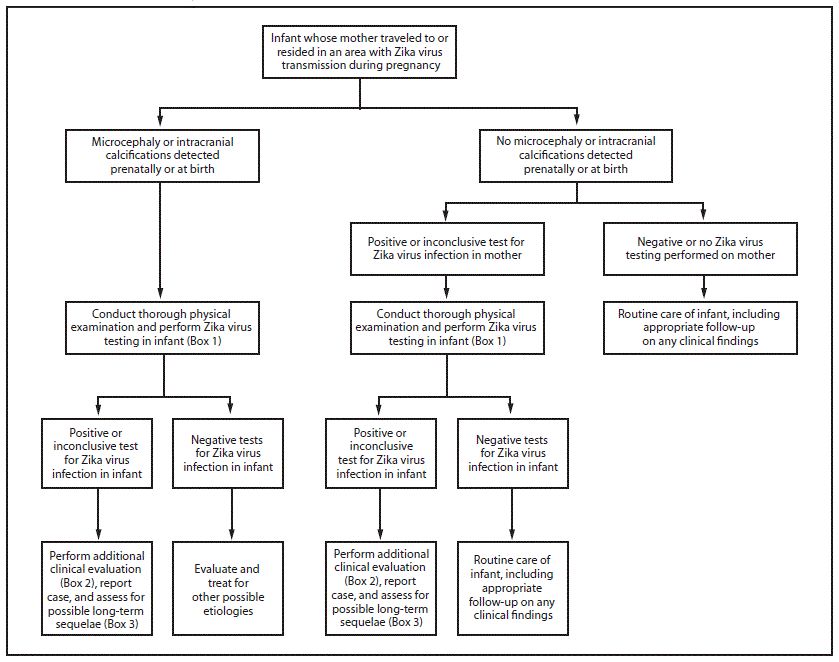 Adapted from: Staples, JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection— United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7.
Adapted from: Staples, JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection— United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7.
*Areas with Zika virus transmission are listed on the CDC website at http://wwwnc.cdc.gov/travel/page/zika-travel-information.
†Microcephaly defined as occipitofrontal circumference less than the third percentile for gestational age and sex based on standard growth curves (26,27), not explained by other etiologies.
§Laboratory evidence of Zika virus infection includes 1) detectable Zika virus, Zika virus RNA, or Zika virus antigen in any clinical specimen; or 2) positive Zika virus IgM with confirmatory neutralizing antibody titers that are ≥4-fold higher than dengue virus neutralizing antibody titers in serum or cerebrospinal fluid. Testing is considered inconclusive if Zika virus neutralizing antibody titers are <4-fold higher than dengue virus neutralizing antibody titers.
¶For infants, perform reverse transcription–polymerase chain reaction (RT-PCR) testing for Zika virus RNA and Zika virus and dengue virus IgM and neutralizing antibodies on serum collected from the umbilical cord or directly from infant within 2 days of birth, if possible. If cerebrospinal fluid is obtained for other reasons, test for Zika virus RNA, Zika virus IgM and neutralizing antibodies, and dengue virus IgM and neutralizing antibodies. Consider histopathologic evaluation of the placenta and umbilical cord with Zika virus immunohistochemical staining on fixed tissue and Zika virus RT-PCR on fixed and frozen tissue. More information on laboratory testing for Zika virus infection is available at http://www.cdc.gov/zika/state-labs/index.html.
 BOX 1. Recommended Zika virus laboratory testing for infants and children when indicated*,†,§
BOX 1. Recommended Zika virus laboratory testing for infants and children when indicated*,†,§
For possible congenital Zika virus infection
- Test infant serum for Zika virus RNA, Zika virus immunoglobulin M (IgM) and neutralizing antibodies, and dengue virus IgM and neutralizing antibodies. The initial sample should be collected either from the umbilical cord or directly from the infant within 2 days of birth, if possible.
- If cerebrospinal fluid is obtained for other studies, test for Zika virus RNA, Zika virus IgM and neutralizing antibodies, and dengue virus IgM and neutralizing antibodies.
- Consider histopathologic evaluation of the placenta and umbilical cord with Zika virus immunohistochemical staining on fixed tissue and Zika virus reverse transcription-polymerase chain reaction (RT-PCR) on fixed and frozen tissue.
- If not already performed during pregnancy, test mother’s serum for Zika virus IgM and neutralizing antibodies, and dengue virus IgM and neutralizing antibodies.
For possible acute Zika virus disease
- If symptoms have been present for <7 days, test serum (and, if obtained for other reasons, cerebrospinal fluid) for Zika virus RNA by RT-PCR
- If Zika virus RNA is not detected and symptoms have been present for ≥4 days, test serum (and, if obtained for other reasons, cerebrospinal fluid) for Zika virus IgM and neutralizing antibodies, and dengue virus IgM and neutralizing antibodies_______________________________
Adapted from: Staples, JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7.
* Indications for testing for congenital infection include 1) an infant with microcephaly or intracranial calcifications born to a woman who traveled to or resided in an area with Zika virus transmission while she was pregnant, or 2) an infant born to a mother with a positive or inconclusive test result for Zika virus infection.
† Indications for testing during acute disease include: Infants and children aged <18 years who 1) traveled to or resided in an affected area within the past 2 weeks and 2) have ≥2 of the following manifestations: fever, rash, conjunctivitis, or arthralgia. Infants in the first 2 weeks of life 1) whose mothers have traveled to or resided in an affected area within 2 weeks of delivery and 2) have ≥2 of the following manifestations: fever, rash, conjunctivitis, or arthralgia.
§ More information on laboratory testing for Zika virus infection is available at http://www.cdc.gov/zika/state-labs/index.html.
 BOX 2. Recommended clinical evaluation and laboratory testing for infants with possible congenital Zika virus infection
BOX 2. Recommended clinical evaluation and laboratory testing for infants with possible congenital Zika virus infection
For all infants with possible congenital Zika virus infection, perform the following:
- Comprehensive physical examination, including careful measurement of occipitofrontal circumference, length, weight, and assessment of gestational age.
- Evaluation for neurologic abnormalities, dysmorphic features, splenomegaly, hepatomegaly, and rash or other skin lesions. Full body photographs and photographic documentation of any rash, skin lesions, or dysmorphic features should be performed. If an abnormality is noted, consultation with an appropriate specialist is recommended.
- Cranial ultrasound, unless prenatal ultrasound results from third trimester demonstrated no abnormalities of the brain.
- Evaluation of hearing by evoked otoacoustic emissions testing or auditory brainstem response testing, either before discharge from the hospital or within 1 month after birth. Infants with abnormal initial hearing screens should be referred to an audiologist for further evaluation.
- Ophthalmologic evaluation, including examination of the retina, either before discharge from the hospital or within 1 month after birth. Infants with abnormal initial eye evaluation should be referred to a pediatric ophthalmologist for further evaluation.
- Other evaluations specific to the infant’s clinical presentation.
For infants with microcephaly or intracranial calcifications, additional evaluation includes the following:
- Consultation with a clinical geneticist or dysmorphologist.
- Consultation with a pediatric neurologist to determine appropriate brain imaging and additional evaluation (e.g., ultrasound, computerized tomography scan, magnetic resonance imaging, and electroencephalogram).
- Testing for other congenital infections such as syphilis, toxoplasmosis, rubella, cytomegalovirus infection, lymphocytic choriomeningitis virus infection, and herpes simplex virus infections. Consider consulting a pediatric infectious disease specialist.
- Complete blood count with platelet count and liver function and enzyme tests, including alanine aminotransferase, aspartate aminotransferase, and bilirubin.
- Consideration of genetic and other teratogenic causes based on additional congenital anomalies that are identified through clinical examination and imaging studies._______________________________
Adapted from: Staples, JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7.
 BOX 3. Recommended long-term follow-up for infants with possible congenital Zika virus infection
BOX 3. Recommended long-term follow-up for infants with possible congenital Zika virus infection
For all infants with possible congenital Zika virus infection, recommended long-term follow-up:
- Report case to state, territorial, or local health department and monitor for additional guidance as it is released.
- Consider conducting additional hearing screen at age 6 months. Refer any child with developmental delay for an audiologic evaluation. Ensure that appropriate follow-up of abnormal newborn hearing screening has occurred.
- Carefully evaluate occipitofrontal circumference and developmental characteristics and milestones throughout the first year of life, in consultation with appropriate medical specialists (e.g., pediatric neurology, developmental and behavioral pediatrics, physical and speech therapy)._______________________________
Adapted from: Staples, JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR Morb Mortal Wkly Rep 2016;65:63–7.
Suggested citation for this article: Fleming-Dutra KE, Nelson JM, Fischer M, et al. Update: Interim Guidelines for Health Care Providers Caring for Infants and Children with Possible Zika Virus Infection — United States, February 2016. MMWR Morb Mortal Wkly Rep 2016;65(Early Release):1–6. DOI: http://dx.doi.org/10.15585/mmwr.mm6507e1er.
CDC: Zika virus disease in the United States, 2015–2016 (January 1, 2015 – February 24, 2016)
Saturday, February 27th, 2016Zika virus disease in the United States, 2015–2016
As of February 24, 2016
- As an arboviral disease, Zika virus is nationally notifiable.
- This update from the CDC Arboviral Disease Branch includes provisional data reported to ArboNET for January 1, 2015 – February 24, 2016.
US States
- Travel-associated Zika virus disease cases reported: 107
- Locally acquired vector-borne cases reported: 0
US Territories
- Travel-associated cases reported: 1
- Locally acquired cases reported: 39
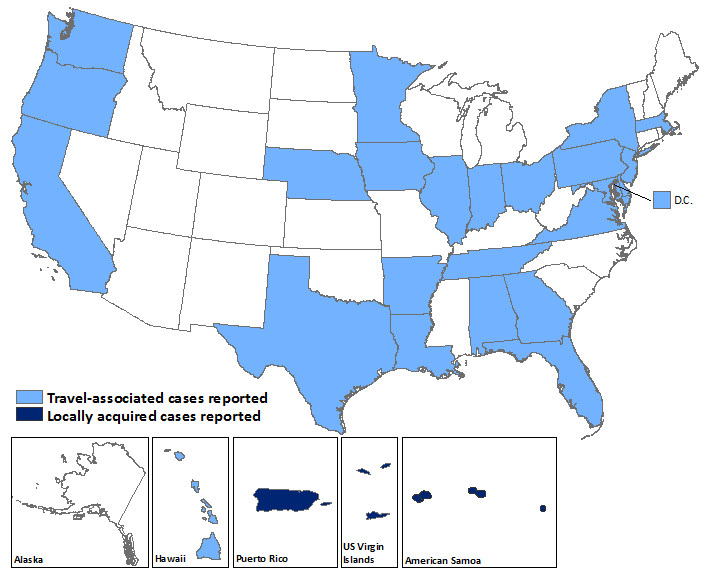
Laboratory-confirmed Zika virus disease cases reported to ArboNET by state or territory — United States, 2015–2016 (as of February 24, 2016)
| States | Travel-associated cases (N=107) | Locally acquired cases (N=0) |
|---|---|---|
| Alabama | 1 | 0 |
| Arkansas | 1 | 0 |
| California | 6 | 0 |
| Delaware | 1 | 0 |
| District of Columbia | 3 | 0 |
| Florida | 28 | 0 |
| Georgia | 1 | 0 |
| Hawaii | 4 | 0 |
| Illinois | 4 | 0 |
| Indiana | 1 | 0 |
| Iowa | 1 | 0 |
| Louisiana | 1 | 0 |
| Maryland | 3 | 0 |
| Massachusetts | 2 | 0 |
| Minnesota | 3 | 0 |
| Nebraska | 2 | 0 |
| New Jersey | 1 | 0 |
| New York | 17 | 0 |
| Ohio | 4 | 0 |
| Oregon | 1 | 0 |
| Pennsylvania | 4 | 0 |
| Tennessee | 1 | 0 |
| Texas | 13 | 0 |
| Virginia | 3 | 0 |
| Washington | 1 | 0 |
| Territories | (N=1) | (N=39) |
| American Samoa | 0 | 4 |
| Puerto Rico | 1 | 34 |
| US Virgin Islands | 0 | 1 |






