Archive for the ‘Chikungunya’ Category
An experimental vaccine to protect against the mosquito-borne illness chikungunya is being tested in a Phase 2 trial
Friday, November 27th, 2015 This digitally-colorized transmission electron micrograph (TEM) depicts numerous chikungunya virus particles, which are composed of a central dense core that is surrounded by a viral envelope.
This digitally-colorized transmission electron micrograph (TEM) depicts numerous chikungunya virus particles, which are composed of a central dense core that is surrounded by a viral envelope.
Credit: CDC
NIH-Sponsored Clinical Trial of Chikungunya Vaccine Opens
An experimental vaccine to protect against the mosquito-borne illness chikungunya is being tested in a Phase 2 trial sponsored by the National Institutes of Health. Results from an initial trial of the vaccine, which was developed by scientists at the NIH National Institute of Allergy and Infectious Diseases (NIAID), were reported in 2014. In that study, all 25 vaccine recipients developed robust immune responses and no safety concerns were noted. The new trial is designed to enroll 400 healthy adult volunteers aged 18 to 60 years old at six sites in the Caribbean. It will continue to gather data on the candidate vaccine’s safety and ability to elicit immune responses, including antibodies.
###
References:
L-J Chang et al. Chikungunya virus-like particle vaccine elicits neutralizing antibodies in healthy adults in a phase I dose escalation clinical trial. The Lancet DOI: 10.1016/S0140-6736(14)61185-5 (2014).
Chikungunya: Current Situation
Saturday, November 21st, 2015Where Has Chikungunya Virus Been Found?
- Prior to 2013, chikungunya virus outbreaks had been identified in countries in Africa, Asia, Europe, and the Indian and Pacific Oceans.
- In late 2013, the first local transmission of chikungunya virus in the Americas was identified in Caribbean countries and territories. Local transmission means that mosquitoes in the area have been infected with the virus and are spreading it to people.
- Since then, local transmission has been identified in 45 countries or territories throughout the Americas with more than 1.7 million suspected cases reported to the Pan American Health Organization from affected areas (Updated data from PAHO).
- For information on current outbreaks, consult CDC’s Traveler’s Health website.
- Click here for more information on chikungunya in the United States(http://www.cdc.gov/chikungunya/geo/united-states.html).
Countries and territories where chikungunya cases have been reported* (as of October 20, 2015)
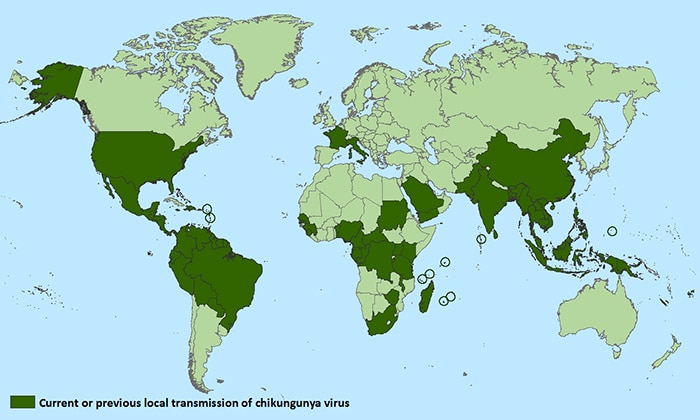
*Does not include countries or territories where only imported cases have been documented. This map is updated weekly if there are new countries or territories that report local chikungunya virus transmission.
AFRICA
- Benin
- Burundi
- Cameroon
- Central African Republic
- Comoros
- Democratic Republic of the Congo
- Equatorial Guinea
- Gabon
- Guinea
- Kenya
- Madagascar
- Malawi
- Mauritius
- Mayotte
- Nigeria
- Republic of Congo
- Reunion
- Senegal
- Seychelles
- Sierra Leone
- South Africa
- Sudan
- Tanzania
- Uganda
- Zimbabwe
ASIA
- Bangladesh
- Bhutan
- Cambodia
- China
- India
- Indonesia
- Laos
- Malaysia
- Maldives
- Myanmar (Burma)
- Pakistan
- Philippines
- Saudi Arabia
- Singapore
- Sri Lanka
- Taiwan
- Thailand
- Timor
- Vietnam
- Yemen
EUROPE
- Italy
- France
AMERICAS
- Anguilla
- Antigua and Barbuda
- Aruba
- Bahamas
- Barbados
- Belize
- Bolivia
- Brazil
- British Virgin Islands
- Cayman Islands
- Colombia
- Costa Rica
- Curacao
- Dominica
- Dominican Republic
- Ecuador
- El Salvador
- French Guiana
- Grenada
- Guadeloupe
- Guatemala
- Guyana
- Haiti
- Honduras
- Jamaica
- Martinique
- Mexico
- Montserrat
- Nicaragua
- Panama
- Paraguay
- Peru
- Puerto Rico
- Saint Barthelemy
- Saint Kitts and Nevis
- Saint Martin
- Sint Maarten
- Saint Lucia
- Saint Vincent and the Grenadines
- Suriname
- Trinidad and Tobago
- Turks and Caicos Islands
- United States
- US Virgin Islands
- Venezuela
OCEANIA/PACIFIC ISLANDS
- American Samoa
- Cook Islands
- Federal States of Micronesia
- French Polynesia
- Kiribati
- New Caledonia
- Papua New Guinea
- Samoa
- Tokelau
- Tonga

