Archive for the ‘Ebola’ Category
Ebola’s survivors: Cataracts
Saturday, October 21st, 2017“….Cataracts usually afflict the old, not the young, but doctors have been shocked to find them in Ebola survivors as young as 5. And for reasons that no one understands, some of those children have the toughest, thickest cataracts that eye surgeons have encountered, along with scarring deep inside the eye….”
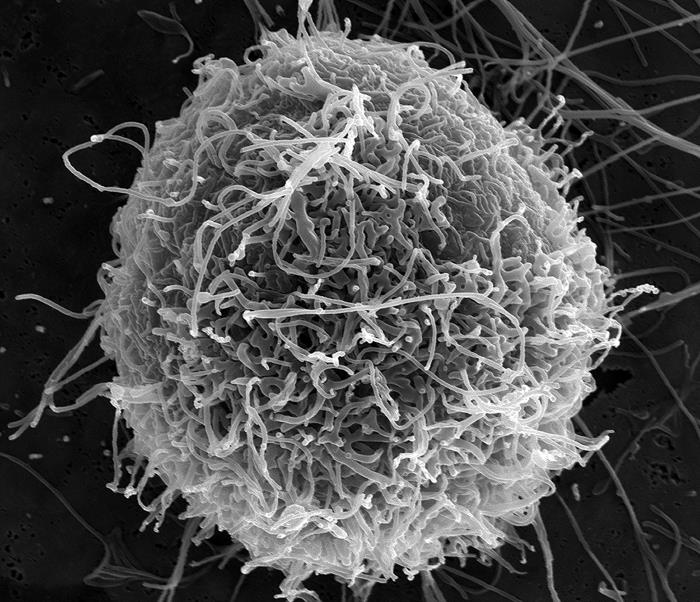
Under a magnification of 25,000X, this scanning electron microscopic (SEM) image depicts numerous filamentous Ebola virus particles budding from a chronically-infected VERO E6 cell.
Predicting Ebola in a patient: Headache, diarrhea, difficulty breathing, nausea and vomiting, loss of appetite, and conjunctivitis. The laboratory tests most useful were creatinine, creatine kinase, alanine aminotransferase, and total bilirubin.
Thursday, October 19th, 2017Oza, S., Sesay, A. A., Russell, N. J., Wing, K., Boufkhed, S., Vandi, L….Checchi, F. (2017). Symptom- and Laboratory-Based Ebola Risk Scores to Differentiate Likely Ebola Infections. Emerging Infectious Diseases, 23(11), 1792-1799. https://dx.doi.org/10.3201/eid2311.170171.
“…..This risk score correctly identified 92% of Ebola-positive patients as high risk for infection; both scores correctly classified >70% of Ebola-negative patients as low or medium risk. Clinicians can use these risk scores to gauge the likelihood of triaged patients having Ebola while awaiting laboratory confirmation…..”
Guidelines focusing on the delivery of supportive care measures to patients in Ebola treatment units where health care resources are limited
Thursday, October 19th, 2017Evidence-based guidelines for supportive care of patients with Ebola virus disease
Lamontagne, François et al.
The Lancet

There is evidence to support that Ebola virus may have a direct role in muscular damage and imbalance of the coagulation system.
Tuesday, August 22nd, 2017“….Though the study provided no evidence that Ebola affected the kidneys, kidney damage is often seen in Ebola patients.
The authors said this was because Ebola viremia was strongly related to evidence of rhabdomyolysis, the rapid breakdown of muscles, which stresses the kidneys….”
Researchers followed 27 Ebola survivors in Sierra Leone for 1 year after diagnosis and found they were seven times more likely than their close contacts to report a disability.
Tuesday, August 22nd, 2017“….Major limitations in vision, mobility, cognition, and affect were observed in survivors one year following the 2014-6 Ebola outbreak, highlighting the need for long-term rehabilitation…..”
The National Ebola Training and Education Center
Wednesday, August 2nd, 2017Abstract
The National Ebola Training and Education Center (NETEC) was established in 2015 in response to the 2014-2016 Ebola virus disease outbreak in West Africa. The US Department of Health and Human Services office of the Assistant Secretary for Preparedness and Response and the US Centers for Disease Control and Prevention sought to increase the competency of healthcare and public health workers, as well as the capability of healthcare facilities in the United States, to deliver safe, efficient, and effective care to patients infected with Ebola and other special pathogens nationwide. NYC Health + Hospitals/Bellevue, Emory University, and the University of Nebraska Medical Center/Nebraska Medicine were awarded this cooperative agreement, based in part on their experience in safely and successfully evaluating and treating patients with Ebola virus disease in the United States. In 2016, NETEC received a supplemental award to expand on 3 initial primary tasks: (1) develop metrics and conduct peer review assessments; (2) develop and provide educational materials, resources, and tools, including exercise design templates; (3) provide expert training and technical assistance; and, to add a fourth task, create a special pathogens clinical research network.
FDA develops rapid and sensitive assay to assess antibody response to Ebola virus vaccine without using the virus
Sunday, July 9th, 2017Scientists at the U.S. Food and Drug Administration (FDA) have developed an assay that assesses the ability of antibodies to neutralize Ebola virus, using a technique that does not require the use of Ebola virus itself and can be automated for rapid testing of large numbers of samples.
The new FDA assay is important because the effectiveness of most licensed viral vaccines is based on their ability to trigger production of neutralizing antibodies. Therefore, methods for assessing neutralization activity of antibodies will likely be an important component for evaluating the effectiveness of Ebola virus vaccines and identifying correlates of protection (measurable signs of immunity).
The assay is based on a widely used technique called micro-neutralization, which measures the ability of antibodies to prevent viruses from infecting animal cells and reproducing themselves. The greater the neutralization of a virus by antibodies, the fewer the number of viruses are able to infect cells and the less the viruses can replicate themselves by making copies of viral genetic material.
A key attribute of the assay is built upon the use of a genetically modified, non-disease-causing virus called vesicular stomatitis virus (VSV). The modified VSV carries part of the genome from Ebola virus and can substitute for Ebola virus in certain assays—an approach previously used at FDA.
The use of genetically engineered VSV eliminates the need for additional precautions, like a BSL-4 laboratory, because the modified virus is incapable of causing Ebola disease. These laboratories are designed for working with pathogens that pose a high risk of life-threatening disease through aerosol transmission and for which there is no vaccine or treatment. The FDA assay is appropriate for BSL-2 laboratories, which are widely available and do not require the more elaborate containment requirements of BSL-4. The need for BSL-4 laboratories for scientists to work with Ebola virus has complicated the worldwide effort to study the virus and develop and assess the effectiveness of Ebola virus vaccines.
The FDA scientists genetically modified different versions of VSV, so each one carried on its surface one of four variations of a molecule called an envelope glycoprotein (GP) found on different strains of the Ebola virus. Then they used a technique called quantitative polymerase chain reaction to measure the amount of genetic material produced by the hybrid VSV after it had been exposed to commercially available antibodies to Ebola virus. Automating the process should offer an important time advantage to public health scientists during investigations of an outbreak. The assay can determine within 6 to 16 hours if antibodies are effective against the Ebola virus.
The scientists showed that the assay was able to assess whether specific antibodies targeting each GP neutralized the different hybrid VSV variants, preventing the virus from infecting the cells and multiplying. Moreover, the results of the Ebola antibody assays agreed with those obtained by other, more complex assays, now used for such testing. This suggests that the assay will be useful in evaluating the ability of antibodies, triggered either by vaccines or natural infection, to neutralize specific varieties of the virus. Moreover, it might be possible to adapt the assay to assess neutralizing antibodies against other viral pathogens.
Title
Development of a micro-neutralization assay for ebolaviruses using a replication-competent vesicular stomatitis hybrid virus and a quantitative PCR readout
Vaccine 17 April 2017
DOI: 10.1016/j.vaccine.2017.03.019![]()
Authors
Stella S. Lee, Kathryn Phy, Keith Peden ⇑, Li Sheng-Fowler
Laboratory of DNA Viruses, Division of Viral Products, Office of Vaccines Research and Review, Center for Biologics Evaluation and Research, Food and Drug Administration, Silver Spring, MD 20993, United States
⇑ Corresponding author at: Building 52/72, Room 1220, CBER, FDA, 10903 New Hampshire Avenue, Silver Spring, MD 20993, United States.
E-mail address: keith.peden@fda.hhs.gov (K. Peden).
Red Cross’ safe and dignified burial (SDB) activities in the three West African outbreak countries prevented from 1,411 to 10,452 secondary Ebola cases
Sunday, June 25th, 2017Tiffany A, Dalziel BD, Kagume Njenge H, Johnson G, Nugba Ballah R, James D, et al. (2017) Estimating the number of secondary Ebola cases resulting from an unsafe burial and risk factors for transmission during the West Africa Ebola epidemic. PLoS Negl Trop Dis 11(6): e0005491. https://doi.org/10.1371/journal.pntd.0005491
“……Red Cross safe and dignified burial (SDB) activities….may have reduced the epidemic by 4.9% to 36.5%…..”
USA: High-level isolation units in select Ebola hospitals report struggling to fund ongoing operations and sustain readiness.
Saturday, June 3rd, 2017Volume 23, Number 6—June 2017
Dispatch
Sustainability of High-Level Isolation Capabilities among US Ebola Treatment Centers
Main Article(https://wwwnc.cdc.gov/eid/article/23/6/17-0062_article)
Figure 1
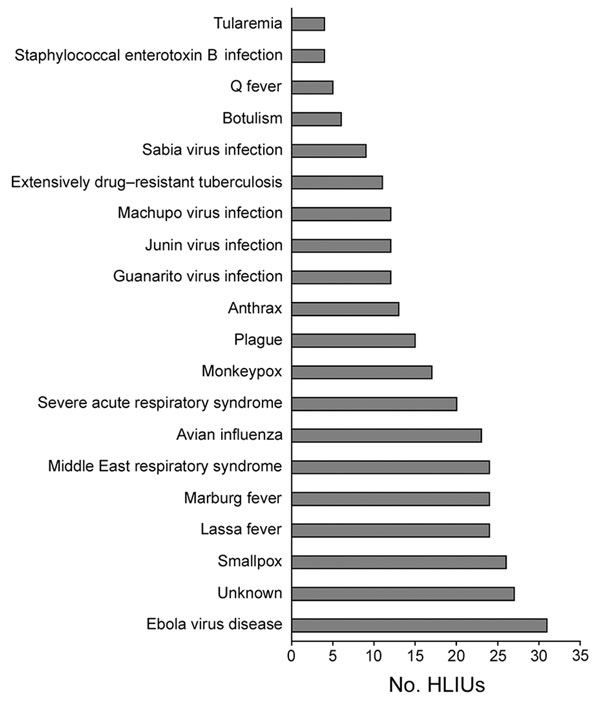
Figure 1. Diseases that 31 HLIUs reported they would treat, United States, 2016. HLIU, high-level isolation unit.
Main Article(https://wwwnc.cdc.gov/eid/article/23/6/17-0062_article)
New technology for rapid diagnosis of Ebola in the Democratic Republic of the Congo
Thursday, June 1st, 2017New technology allows for rapid diagnosis of Ebola in the Democratic Republic of the Congo
Laboratory testing of samples is essential to rapidly assess the scope and spread of any Ebola outbreak. Since the major outbreak in West Africa in 2014, an increasing number of diagnostic tools have become available to perform rapid initial testing of samples. The Democratic Republic of the Congo is using these new tools, as well as classic ones, to respond to an ongoing outbreak of the virus in a very remote area of the north east of the country.

A small cluster of undiagnosed illness and deaths with haemorrhagic signs was reported in the Province of Bas-Uele in early May. Congolese scientists quickly gathered samples, shipped them to Kinshasa and tested them at the National Institute of Biomedical Research (INRB). The results, subsequently confirmed by the Centre International de Recherche Médicale de Franceville (CIRMF), a WHO collaborating centre in Gabon, showed this is an outbreak due to Ebola virus disease (Ebolazaire).

As soon as the outbreak was detected, the Ministry of Health, together with WHO and other partners, mobilized laboratory resources to ensure investigations could be conducted as quickly as possible to guide the response. In addition to the testing facilities available at the INRB in Kinshasa, an INRB mobile field lab was quickly dispatched to the affected health zone of Likati.
To control the outbreak, multi-disciplinary field teams in Likati have been actively searching for suspect cases. Anyone presenting with certain pre-defined symptoms, such as sudden onset of fever and/or unexplained bleeding, is considered a suspect Ebola case until laboratory results prove otherwise. Discarding suspect cases that test negative for Ebola allows response teams to focus on tracing only the contacts of those who have either tested positive or whose status is unknown.
The MOH, WHO and partners have rapidly set up an intensified field alert and response system in Likati. This is resulting in early identification of suspect cases detected in the affected zone. The field laboratory provides the capability to rapidly test samples on site and focus support and follow-up on any new laboratory confirmed cases and contacts. In this extremely remote and challenging area, this mobile lab is providing a core element of robust surveillance, which is essential to bringing this outbreak to an end as quickly as possible.

One of the technologies being used to detect Ebola in DRC is GeneXpert, which was primarily developed to detect cases of tuberculosis, but has been adapted to enable rapid testing of many pathogens – HIV, malaria, STIs, and Ebola. At the INRB laboratory in Kinshasa – with support from USAID, WHO, Canada, the Global Outbreak Alert and Response Network (GOARN) and the Emerging and Dangerous Pathogens Laboratory Network (EDPLN) – technicians can use GeneXpert to test for the Zaire strain of Ebola in just one hour. For samples that are negative, further testing is then undertaken to check for other strains of Ebola, other viral haemorrhagic fevers, or other diseases.

Other tests developed during the West African outbreak are also being deployed, such as OraQuick — a rapid diagnostic test, which has been developed with the support of the US Centers for Disease Control and Prevention and GOARN. In the field, OraQuick can test blood or saliva samples for Ebola in just half an hour.
Even if many or all suspect cases now being tested are negative, it remains vital to actively follow contacts of all confirmed, probable, and suspect cases for 21 days, and then to continue enhanced surveillance for an additional 21-day period. Any period of calm is an opportunity to continue building and reinforcing local and country preparedness and response capacities and ensuring rapid investigation teams are ready in case the virus should resurface.
This is the eighth outbreak of Ebola virus disease in the Democratic Republic of the Congo since the disease was discovered in the 1970s in DRC. Health authorities in this country are recognized throughout the African region and the world as experts in responding to outbreaks of this disease.