Recommendations for the Use of Influenza Vaccines, 2017–18 Season
Groups Recommended for Vaccination
Routine annual influenza vaccination is recommended for all persons aged ≥6 months who do not have contraindications. Recommendations regarding timing of vaccination, considerations for specific populations, the use of specific vaccines, and contraindications and precautions are summarized in the sections that follow.
Timing of Vaccination
Optimally, vaccination should occur before onset of influenza activity in the community. Health care providers should offer vaccination by the end of October, if possible. Children aged 6 months through 8 years who require 2 doses (see Children Aged 6 Months through 8 Years) should receive their first dose as soon as possible after vaccine becomes available, to allow the second dose (which must be administered ≥4 weeks later) to be received by the end of October. Although some available data indicate that early vaccination (e.g., in July and August) might be associated with suboptimal immunity before the end of the influenza season, particularly among older adults, the relative contribution of potential waning of immunity compared with those of other determinants of the impact of vaccination (e.g., timing and severity of the influenza season, the impact of missed opportunities when individuals delay vaccination and fail to return later in the season, and programmatic constraints) is unknown. Although delaying vaccination might result in greater immunity later in the season, deferral also might result in missed opportunities to vaccinate, as well as difficulties in vaccinating a population within a more constrained time period. Community vaccination programs should balance maximizing likelihood of persistence of vaccine-induced protection through the season with avoiding missed opportunities to vaccinate or vaccinating after onset of influenza circulation occurs. Revaccination later in the season of persons who have already been fully vaccinated is not recommended.
Vaccination should continue to be offered as long as influenza viruses are circulating and unexpired vaccine is available. To avoid missed opportunities for vaccination, providers should offer vaccination during routine health care visits and hospitalizations when vaccine is available. Vaccination efforts should be structured to ensure the vaccination of as many persons as possible before influenza activity in the community begins.
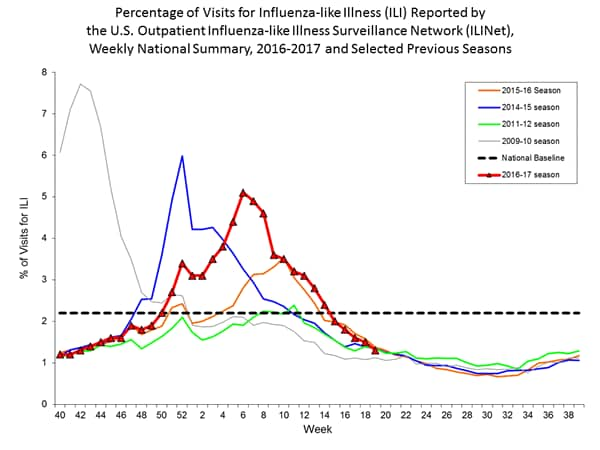
In any given season, the optimal time to vaccinate cannot be predicted precisely because influenza seasons vary in timing and duration. Moreover, more than one outbreak might occur in a given community in a single year. In the United States, localized outbreaks that indicate the start of seasonal influenza activity can occur as early as October. However, in 74% of influenza seasons from 1982–83 through 2015–16, peak influenza activity (which often is close to the midpoint of influenza activity for the season) has not occurred until January or later, and in 59% of seasons, the peak was in February or later (10).
In recent seasons, initial shipments of influenza vaccine have arrived to some vaccine providers as early as July. Very early availability of vaccine as compared with typical onset and peak of influenza activity raises questions related to the ideal time to begin vaccination. Several observational studies of influenza vaccine effectiveness have reported decreased vaccine protection within a single season, particularly against influenza A(H3N2) (11–14). In some of these studies decline in VE was particularly pronounced among older adults (12,13). Some studies have documented decline in protective antibodies over the course of one season (15–17), with antibody levels decreasing with greater time elapsed postvaccination. However, the rate and degree of decline observed has varied. Among adults in one study, HA and neuraminidase antibody levels declined slowly, with a two-fold decrease in titer estimated to take >600 days (18). A review of studies reporting postvaccination seroprotection rates among adults aged ≥60 years noted that seroprotection levels meeting Committee of Proprietary Medicinal Products standards were maintained for ≥4 months for the H3N2 component in all 8 studies and for the H1N1 and B components in five of seven studies (19). A recent multiseason analysis from the U.S. Influenza Vaccine Effectiveness (U.S. Flu VE) Network found that VE declined by about 7% per month for H3N2 and influenza B, and 6%–11% per month for H1N1pdm09 (20). VE remained greater than zero for at least 5 to 6 months after vaccination. Similar waning effects have not been observed consistently across age groups and virus subtypes in different populations, and the observed decline in protection could be attributable to bias, unmeasured confounding, or the late season emergence of antigenic drift variants that are less well-matched to the vaccine strain.
Vaccination efforts should continue throughout the season because the duration of the influenza season varies and influenza activity might not occur in certain communities until February or March. Providers should offer influenza vaccine routinely, and organized vaccination campaigns should continue throughout the influenza season, including after influenza activity has begun in the community. Although vaccination by the end of October is recommended, vaccine administered in December or later, even if influenza activity has already begun, is likely to be beneficial in the majority of influenza seasons.
Guidance for Use in Specific Populations and Situations
Populations at Higher Risk for Medical Complications Attributable to Severe Influenza
All persons aged ≥6 months without contraindications should be vaccinated annually. However, vaccination to prevent influenza is particularly important for persons who are at increased risk for severe complications from influenza and for influenza-related outpatient, ED, or hospital visits. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to the following persons at increased risk for medical complications attributable to severe influenza who do not have contraindications (no hierarchy is implied by order of listing):
- all children aged 6 through 59 months;
- all persons aged ≥50 years;
- adults and children who have chronic pulmonary (including asthma) or cardiovascular (except isolated hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus);
- persons who are immunocompromised due to any cause (including immunosuppression caused by medications or by HIV infection);
- women who are or will be pregnant during the influenza season;
- children and adolescents (aged 6 months through 18 years) who are receiving aspirin- or salicylate-containing medications and who might be at risk for experiencing Reye syndrome after influenza virus infection;
- residents of nursing homes and other long-term care facilities;
- American Indians/Alaska Natives; and
- persons who are extremely obese (BMI ≥40).
ACIP recommends that LAIV4 not be used during the 2017–18 season for any population. Providers who elect to use it should consider previous guidance for use of LAIV4 for high-risk populations (Table 2).
Persons Who Live With or Care for Persons at Higher Risk for Influenza-Related Complications
All persons aged ≥6 months without contraindications should be vaccinated annually; however, continued emphasis should be placed on vaccination of persons who live with or care for persons at higher risk for influenza-related complications. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to persons at higher risk for influenza-related complications listed above, as well as these persons:
- health care personnel, including physicians, nurses, and other workers in inpatient and outpatient-care settings, medical emergency-response workers (e.g., paramedics and emergency medical technicians), and employees of nursing home and long-term care facilities who have contact with patients or residents, and students in these professions who will have contact with patients. ACIP guidance for immunization of health care personnel has been published previously (21);
- household contacts (including children) and caregivers of children aged ≤59 months (i.e., aged <5 years) and adults aged ≥50 years, particularly contacts of children aged <6 months; and
- household contacts (including children) and caregivers of persons with medical conditions that put them at high risk for severe complications from influenza.
ACIP recommends that LAIV4 not be used during the 2017–18 season for any population. Providers who elect to use it should consider previous guidance for use of LAIV4 for persons who care for or have contact with immunocompromised persons (22). Health care personnel and persons who are contacts of persons in these groups and who are contacts of severely immunocompromised persons (those living in a protected environment) may receive any IIV or RIV that is otherwise indicated. ACIP and HICPAC have previously recommended that health care personnel who receive LAIV should avoid providing care for severely immunosuppressed patients requiring a protected environment for 7 days after vaccination, and that hospital visitors who have received LAIV4 should avoid contact with severely immunosuppressed persons (i.e., persons requiring a protected environment) for 7 days after vaccination. However, such persons should not be restricted from visiting less severely immunosuppressed patients.
Children Aged 6 Months Through 8 Years
Dose volume for children aged 6 through 35 months: Children aged 6 through 35 months may receive one of two products at the appropriate volume for each dose needed: 0.5 mL FluLaval Quadrivalent (containing 15 µg of HA per vaccine virus) or 0.25 mL Fluzone Quadrivalent (containing 7.5 µg of HA per vaccine virus). These are the only two influenza vaccine products licensed for this age group. Care should be taken to administer the appropriate volume for each needed dose of either product. In either instance, the needed volume may be administered from an appropriate prefilled syringe, a single dose vial, or multidose vial, as supplied by the manufacturer. Note, however, that if a 0.5 mL single-use vial of Fluzone Quadivalent is used for a child aged 6 through 35 months, only half the volume should be administered and the other half should be discarded.
Before November 2016, the only influenza vaccine formulations licensed for children aged 6 through 35 months were the 0.25 mL (containing 7.5 µg of HA per vaccine virus) dose formulations of Fluzone and Fluzone Quadrivalent. The recommendation for use of a reduced dose volume for children in this age group (half that recommended for persons aged ≥3 years) was based on increased reactogenicity noted among children (particularly younger children) following receipt of influenza vaccines in trials conducted during the 1970s. This increased reactogenicity was primarily observed with whole-virus inactivated vaccines (23–27). Studies with vaccines more similar to currently available split-virus inactivated products demonstrated less reactogenicity (27). Recent comparative studies of 0.5 mL vs. 0.25 mL doses of IIV3 conducted among children aged 6 through 23 months (28) and 6 through 35 months (29) noted no significant difference in reactogenicity at the higher dose. In a randomized trial comparing immunogenicity and safety of 0.5 mL FluLaval Quadrivalent with 0.25 mL Fluzone Quadrivalent, safety and reactogenicity were similar between the two vaccines. In a post-hoc analysis, superior immunogenicity was noted for the B components of FluLaval Quadrivalent among infants aged 6 through 17 months and for unprimed children (those who had not previously received at least 2 doses of influenza vaccine) aged 6 through 35 months (30).
Number of doses for children aged 6 months through 8 years: Evidence from several studies indicates that children aged 6 months through 8 years require 2 doses of influenza vaccine (administered a minimum of 4 weeks apart) during their first season of vaccination for optimal protection (31–34). Children aged 6 months through 8 years who have previously received ≥2 total doses of trivalent or quadrivalent influenza vaccine before July 1, 2017 require only 1 dose for 2017–18. The 2 doses of influenza vaccine do not have to have been administered in the same season or consecutive seasons. Children in this age group who have not previously received ≥2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2017 require 2 doses for the 2017–18 season. The interval between the 2 doses should be at least 4 weeks (Figure).
Pregnant Women
Because pregnant and postpartum women are at higher risk for severe illness and complications from influenza than women who are not pregnant, ACIP recommends that all women who are pregnant or who might be pregnant in the influenza season receive influenza vaccine. Any licensed, recommended, and age-appropriate influenza vaccine may be used. Influenza vaccine can be administered at any time during pregnancy, before and during the influenza season. ACIP recommends that LAIV4 not be used in any population for the 2017–18 season. Providers should note that, as a live virus vaccine, LAIV4 should not be used during pregnancy.
Although experience with the use of IIVs is substantial, and data from observational studies are available to support the safety of these vaccines in pregnancy, data are more limited for vaccination during the first trimester (see Safety of Influenza Vaccines: Pregnant Women and Neonates in the Background Document). Moreover, there is substantially less experience with more recently licensed IIV products (e.g., quadrivalent, cell culture-based, and adjuvanted vaccines) during pregnancy in general. For RIV (available as RIV3 since the 2013–14 influenza season, and as RIV3 and RIV4 for 2017–18), data are limited to reports of pregnancies occurring incidentally during clinical trials, VAERS reports, and pregnancy registry reports. Pregnancy registries and surveillance studies exist for some products; information may be found in package inserts (35–42), available at https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm094045.htm for trivalent vaccines and https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm295057.htm for quadrivalent vaccines.
Older Adults
For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV are acceptable options. Fluzone High-Dose (HD-IIV3; Sanofi Pasteur, Swiftwater, Pennsylvania) met prespecified criteria for superior efficacy to that of SD-IIV3 in a randomized trial conducted over two seasons among 31,989 persons aged ≥65 years, and might provide better protection than SD-IIV3 for this age group (43–45). In an exploratory analysis of data from a single-season randomized trial conducted among 8,604 adults aged ≥50 years, Flublok Quadrivalent (RIV4; Protein Sciences, Meriden, Connecticut) was more efficacious than SD-IIV4 (46,47); however, no claim of superiority was approved for the package insert (47). Fluad (aIIV3; Seqirus, Holly Springs, North Carolina) was more effective against laboratory-confirmed influenza than unadjuvanted SD-IIV3 among adults aged ≥65 years (N = 227) in an analysis from a small observational study (48). No preferential recommendation is made for any specific vaccine product. Vaccination should not be delayed if a specific product is not readily available.
Because of the vulnerability of this population to severe influenza illness, hospitalization, and death, efficacy and effectiveness of influenza vaccines among older adults is an area of active research (see Immunogenicity, Efficacy, and Effectiveness of Influenza Vaccines: HD-IIV3, aIIV3, and RIV4 for Older Adults in the Background Document). Recent comparative studies of efficacy/effectiveness against laboratory-confirmed influenza outcomes among older adults have focused on HD-IIV3 (Fluzone High Dose; Sanofi Pasteur, Swiftwater, Pennsylvania) (43,49–51), aIIV3 (Fluad, Seqirus, Holly Springs, North Carolina) (48), and RIV4 (Flublok Quadrivalent; Protein Sciences, Meriden, Connecticut) (46). Characteristics of these studies are summarized (Table 3). In each instance, the comparator vaccines have been standard dose, inactivated vaccines (SD-IIV3 as the comparator for HD-IIV3 and aIIV3; SD-IIV4 as the comparator for RIV4). No data are yet available from studies comparing the efficacy or effectiveness of HD-IIV3, aIIV3, and RIV4 with one another among older adults. This lack of comparative data prevents recommending one of these three vaccines over another for this population. HD-IIV3 exhibited superior efficacy over a comparator standard-dose IIV3 for adults aged ≥65 years in a large (N = 31,989), two-season randomized, controlled, double-blind trial (43,44), and might provide better protection than SD-IIV3s for this age group. Additional data concerning relative efficacy of HD-IIV3 for other clinical outcomes, as well as cost-effectiveness analyses and observational studies, are summarized in the Background Document. In a single-season randomized, controlled, double-blind trial comparing RIV4 with a standard-dose unadjuvanted IIV4 among adults aged ≥50 years (N = 8,604), RIV4 was more effective; however, approval for a claim of superiority was not made as a result of this exploratory analysis (46,47). Additional data, including discussion of immunogenicity studies, are described in the Background Document. Fluad (aIIV3; Seqirus, Holly Springs, North Carolina) was more effective against laboratory-confirmed influenza than unadjuvanted SD-IIV3 among adults aged ≥65 years (N = 227) in an analysis from a small observational study (48); no data are yet available concerning efficacy of Fluad compared with nonadjuvanted IIV3 against laboratory-confirmed influenza outcomes from a randomized trial in this population. Additional data concerning aIIV3, from studies examining immunogenicity and non-laboratory confirmed influenza outcomes, are discussed in the Background Document. ACIP will continue to review data concerning the efficacy and effectiveness of these vaccines as more information emerges.
Immunocompromised Persons
Immunocompromised states comprise a heterogeneous range of conditions. In many instances, limited data are available regarding the use of influenza vaccines in the setting of specific immunocompromised states. ACIP recommends that LAIV4 not be used in any population for the 2017–18 season; providers considering its use should note that live virus vaccines should not be used for persons with most forms of altered immunocompetence (52), given the uncertain but biologically plausible risk for disease attributable to the vaccine virus. In addition to potential safety issues, immune response to live or inactivated vaccines might be blunted in some clinical situations, such as for persons with congenital immune deficiencies, persons receiving cancer chemotherapy, and persons receiving immunosuppressive medications. For this reason, timing of vaccination might be a consideration (e.g., vaccinating during some period either before or after an immunocompromising intervention).
The Infectious Diseases Society of America (IDSA) has published detailed guidance for the selection and timing of vaccines for persons with specific immunocompromising conditions, including congenital immune disorders, stem cell and solid organ transplant, anatomic and functional asplenia, and therapeutic drug-induced immunosuppression, as well as for persons with cochlear implants or other conditions leading to persistent cerebrospinal fluid-oropharyngeal communication (53). ACIP will continue to review accumulating data on use of influenza vaccines in these contexts.
Persons with a History of Guillain-Barré Syndrome Following Influenza Vaccination
A history of Guillain-Barré Syndrome (GBS) within 6 weeks following a previous dose of any type of influenza vaccine is considered a precaution to vaccination (Table 2). Persons who are not at high risk for severe influenza complications (see Populations at Higher Risk for Medical Complications Attributable to Severe Influenza) and who are known to have experienced GBS within 6 weeks of a previous influenza vaccination generally should not be vaccinated. As an alternative to vaccination, physicians might consider using influenza antiviral chemoprophylaxis for these persons (54). However, the benefits of influenza vaccination might outweigh the risks for certain persons who have a history of GBS and who also are at high risk for severe complications from influenza.
Persons with a History of Egg Allergy
As is the case for other vaccines, influenza vaccines contain various different components that might cause allergic and anaphylactic reactions. Not all such reactions are related to egg proteins; however, the possibility of reactions to influenza vaccines in egg-allergic persons might be of concern to these persons and vaccine providers. Currently available influenza vaccines, with the exceptions of RIV3, RIV4 and ccIIV4, are prepared by propagation of virus in embryonated eggs. Only RIV3 and RIV4 are considered egg-free. For ccIIV4 (Flucelvax Quadrivalent; Seqirus, Holly Springs, North Carolina), ovalbumin is not directly measured. During manufacture of ccIIV4, viruses are propagated in mammalian cells rather than in eggs; however, some of the viruses provided to the manufacturer are egg-derived, and therefore egg proteins may potentially be introduced at the start of the manufacturing process. Once these viruses are received by the manufacturer, no eggs are used, and dilutions at various steps during the manufacturing process result in a theoretical maximum of 5×10-8 μg/0.5 mL dose of total egg protein (Seqirus, unpublished data, 2016).
Severe allergic reactions to vaccines, although rare, can occur at any time, despite a recipient’s allergy history. Therefore, all vaccine providers should be familiar with the office emergency plan, and be certified in cardiopulmonary resuscitation (52). For persons who report a history of egg allergy, ACIP recommends the following (based upon the recipient’s previous symptoms after exposure to egg):
- Persons with a history of egg allergy who have experienced only urticaria (hives) after exposure to egg should receive influenza vaccine. Any licensed and recommended influenza vaccine (i.e., any IIV or RIV) that is otherwise appropriate for the recipient’s age and health status may be used.
- Persons who report having had reactions to egg involving symptoms other than urticaria (hives), such as angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention, may similarly receive any licensed and recommended influenza vaccine (i.e., any IIV or RIV) that is otherwise appropriate for the recipient’s age and health status. The selected vaccine should be administered in an inpatient or outpatient medical setting (including, but not necessarily limited to, hospitals, clinics, health departments, and physician offices). Vaccine administration should be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
- A previous severe allergic reaction to influenza vaccine, regardless of the component suspected of being responsible for the reaction, is a contraindication to future receipt of the vaccine.
No period of postvaccination observation period is recommended specifically for egg-allergic persons. However, ACIP recommends that vaccine providers consider observing patients for 15 minutes following administration of any vaccine to decrease the risk for injury should syncope occur (52).
Persons who are able to eat lightly cooked egg (e.g., scrambled egg) without reaction are unlikely to be allergic. Egg-allergic persons might tolerate egg in baked products (e.g., bread or cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs and egg-containing foods, plus skin and/or blood testing for immunoglobulin E directed against egg proteins (55).
Occasional cases of anaphylaxis in egg-allergic persons have been reported to VAERS after administration of influenza vaccines (56,57). ACIP will continue to review available data regarding anaphylaxis cases following influenza vaccines.
Vaccination Issues for Travelers
Travelers who want to reduce the risk for influenza infection should consider influenza vaccination, preferably at least 2 weeks before departure. In particular, persons residing in the United States who are at high risk for complications of influenza and who were not vaccinated with influenza vaccine during the preceding Northern Hemisphere fall or winter should consider receiving influenza vaccine before departure if they plan to travel:
- to the tropics,
- with organized tourist groups or on cruise ships, or
- to the Southern Hemisphere during the Southern Hemisphere influenza season (April–September).
No information is available indicating a benefit to revaccinating persons before summer travel who already were vaccinated during the preceding fall. In many cases, revaccination will not be feasible as Southern Hemisphere formulations of influenza vaccine are not generally available in the United States. Persons at high risk who receive the previous season’s vaccine before travel should receive the current vaccine the following fall or winter. Persons at higher risk for influenza complications should consult with their health care practitioner to discuss the risk for influenza or other travel-related diseases before embarking on travel during the summer.
In temperate climate regions of the Northern and Southern hemispheres, influenza activity is seasonal, occurring approximately from October through May in the Northern Hemisphere and April through September in the Southern Hemisphere. In the tropics, influenza occurs throughout the year. Travelers can be exposed to influenza when travelling to an area where influenza is circulating, or when traveling as part of large tourist groups (e.g., on cruise ships) that include persons from areas of the world in which influenza viruses are circulating (58,59). In a survey of Swiss travelers to tropical and subtropical countries, among 211 who reported febrile illness during or after travelling abroad and who provided paired serum samples, 40 demonstrated serologic evidence of influenza infection (60). Among 109 travelers returning to Australia from travel in Asia who reported acute respiratory infection symptoms, four (3.7%) had evidence of influenza A infection (evidenced by fourfold rise in antibody titer) (61).
Influenza vaccine formulated for the Southern Hemisphere might differ in viral composition from the Northern Hemisphere vaccine. However, with the exception of the Southern Hemisphere formulation of Fluzone Quadrivalent (IIV4; Sanofi Pasteur, Swiftwater, Pennsylvania), Southern Hemisphere formulation seasonal influenza vaccines are not licensed in the United States, and Southern Hemisphere formulations generally are not commercially available in the United States. More information on influenza vaccines and travel is available at https://www.cdc.gov/flu/travelers/travelersfacts.htm.
Use of Influenza Antiviral Medications
Administration of IIV or RIV to persons receiving influenza antiviral medications for treatment or chemoprophylaxis is acceptable. ACIP recommends that LAIV4 should not be used during the 2017–18 season. If used, providers should note that influenza antiviral medications may reduce the effectiveness of LAIV4 if given within 48 hours before to 14 days after LAIV4 (62). Persons who receive influenza antiviral medications during this period surrounding receipt of LAIV4 may be revaccinated with another appropriate vaccine formulation (e.g., IIV or RIV).
Concurrent Administration of Influenza Vaccine with Other Vaccines
Data regarding potential interference following simultaneous or sequential administration for the many potential combinations of vaccines are limited. Therefore, following the ACIP General Best Practice Guidelines for Immunization is prudent (52). IIVs and RIV may be administered concurrently or sequentially with other inactivated vaccines or with live vaccines. LAIV4 is not recommended for use in 2017–18. Providers considering its use should note that although inactivated or live vaccines can be administered simultaneously with LAIV4, after administration of a live vaccine (such as LAIV4), at least 4 weeks should pass before another live vaccine is administered.
Relatively limited data are available on the concurrent administration of influenza vaccines with other vaccines. In a study comparing the immunogenicity of IIV and zoster vaccine given either concurrently or separated by a 4-week interval to adults aged ≥50 years, antibody responses were similar for both schedules (63). In some studies, reduced responses have been noted to PCV13 (64,65), tetanus antigens (66), and pertussis antigens (66) when co-administered with IIV; in most instances the clinical significance of this is uncertain. Reassuring safety profiles have been noted for simultaneous administration of zoster vaccine (63), PCV13 (64,65), PPSV23 (67) and Tdap (66) among adults and of Tdap among pregnant women (68). Increased prevalence of local and/or systemic adverse reactions have been noted with concurrent administration in some of these studies, but these symptoms have generally been reported to be mild or moderate. Among children, co-administration of IIV and PCV13 was associated with increased risk of fever on the day of vaccination and the day following (i.e., days 0–1 postvaccination) after vaccination in children aged 6 through 23 months in a study conducted during the 2011–12 season (69). Increased risk of febrile seizure in this age group has been noted within days 0–1 following coadministration of IIV with PCV7, PCV13, or DTaP-containing vaccines during the 2006–07 through 2010–11 seasons (70), and with PCV13 during the 2014–15 season (71). No changes in the recommendations for administration of these vaccines were made, and these vaccines may be given concomitantly. Surveillance of febrile seizures is ongoing through VAERS, and the Vaccine Safety Datalink annual influenza vaccine safety surveillance includes monitoring for seizures following vaccination.
Concurrent administration to children of LAIV3 with MMR and varicella vaccine was not associated with diminished immunogenicity to antigens in any of the vaccines in one study (72); diminished response to rubella was observed in another examining coadministraion of LAIV3 and MMR (73). Administration of OPV was not associated with interference when administered with LAIV (74). No safety concerns were revealed in these studies.