Archive for the ‘Influenza’ Category
2017-2018 Influenza Season Week 5 ending February 3, 2018
Saturday, February 10th, 2018Synopsis:
During week 5 (January 28-February 3, 2018), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 5 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories remained elevated.
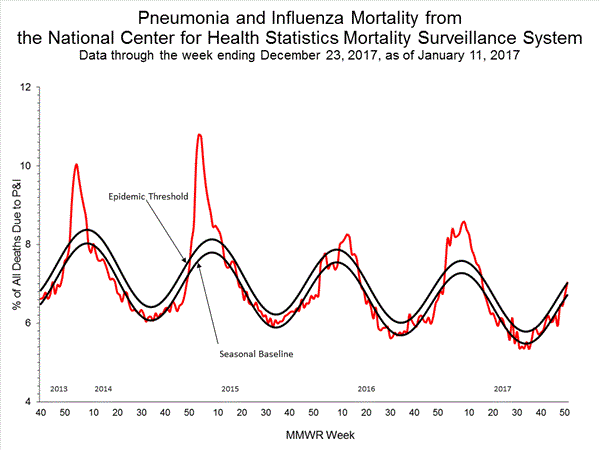
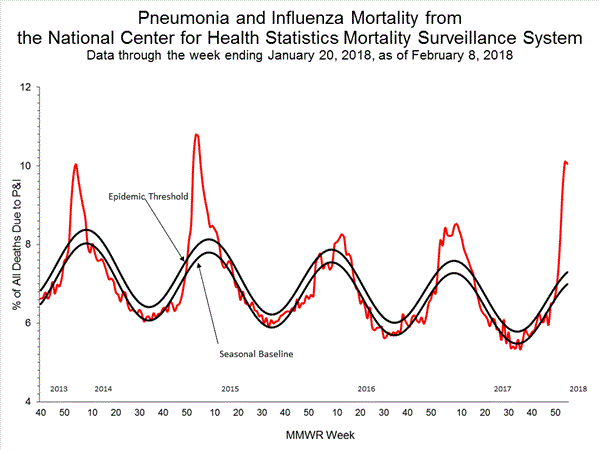
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
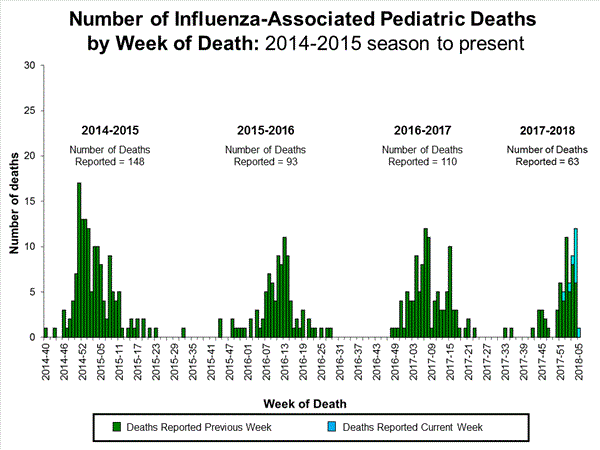
- Influenza-associated Pediatric Deaths: Ten influenza-associated pediatric deaths were reported.
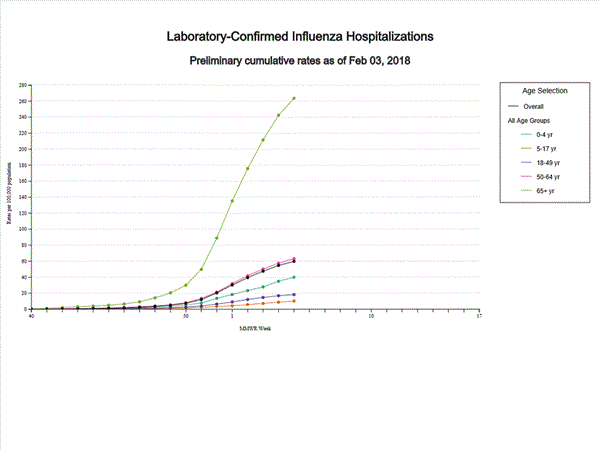
- Influenza-associated Hospitalizations: A cumulative rate of 59.9 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
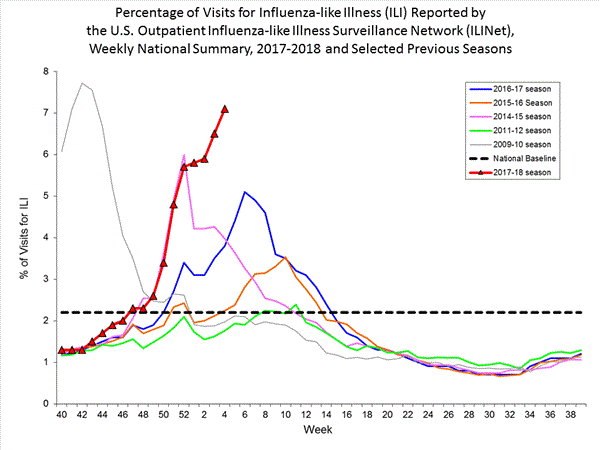
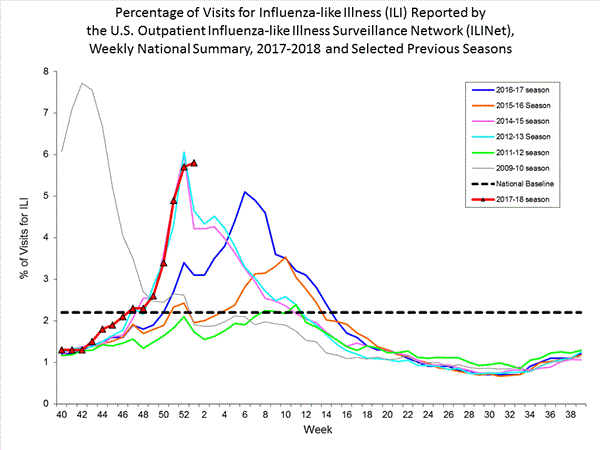
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 7.7%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, the District of Columbia, Puerto Rico and 43 states experienced high ILI activity; three states experienced moderate ILI activity; two states experienced low ILI activity; and two states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 48 states was reported as widespread; two states reported regional activity; the District of Columbia and Guam reported local activity; and the U.S. Virgin Islands reported sporadic activity.
2017-2018 Influenza Season Week 4 ending January 27, 2018
Saturday, February 3rd, 2018Synopsis:
During week 4 (January 21-27, 2018), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 4 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories remained elevated.
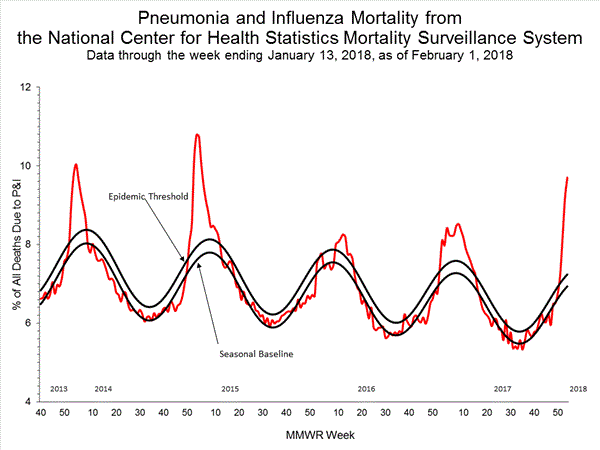
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
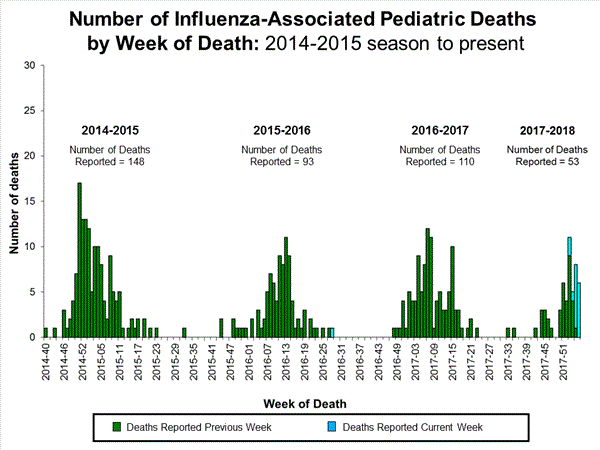
- Influenza-associated Pediatric Deaths: Seventeen influenza-associated pediatric deaths were reported, one of which occurred during the 2015-2016 season.
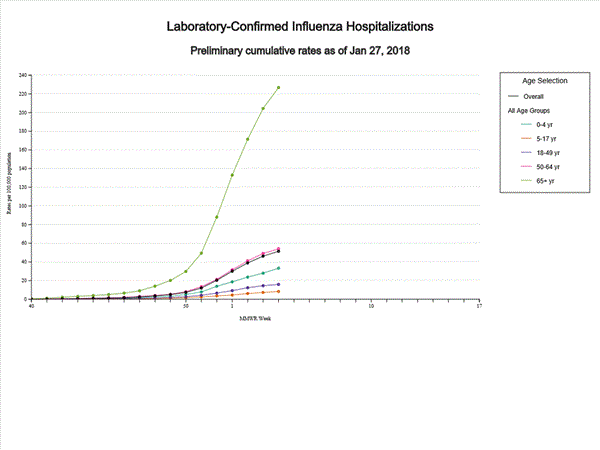
- Influenza-associated Hospitalizations: A cumulative rate of 51.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
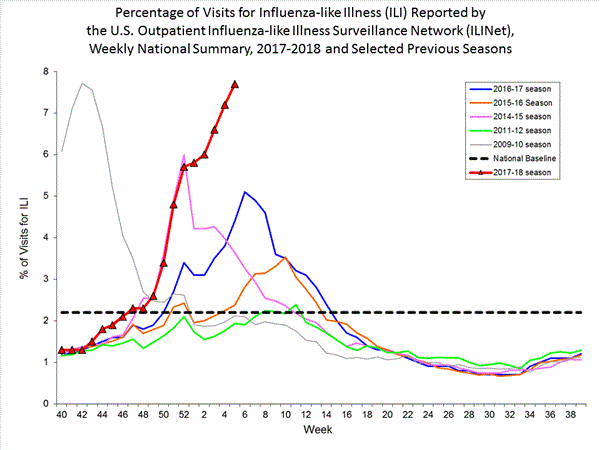
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 7.1%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, the District of Columbia, and 42 states experienced high ILI activity; Puerto Rico and two states experienced moderate ILI activity; three states experienced low ILI activity; and three states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 48 states was reported as widespread; Guam and one state reported regional activity; the District of Columbia and one state reported local activity; and the U.S. Virgin Islands reported sporadic activity.
Influenza-Associated Pediatric Mortality:
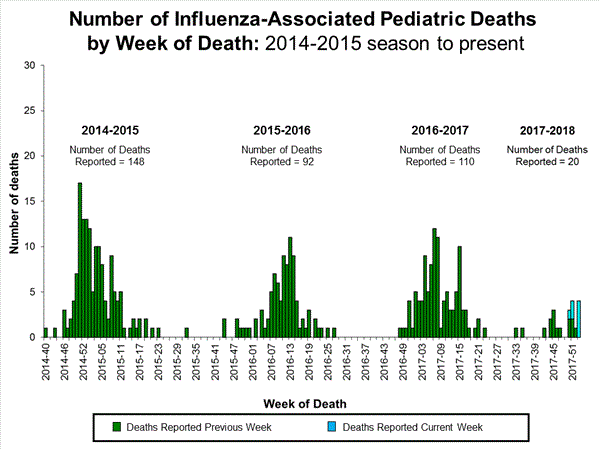
Seventeen influenza-associated pediatric deaths were reported to CDC during week 4.
Five deaths were associated with an influenza A(H3) virus and occurred during weeks 1, 2, 3, and 4 (the weeks ending January 6, January 13, January 20, and January 27, 2018). Two deaths were associated with an influenza A(H1N1)pdm09 virus and occurred during weeks 3 and 4 (the weeks ending January 20, 2018, and January 27, 2018, respectively). Four deaths were associated with an influenza A virus for which no subtyping was performed and occurred during weeks 3 and 4. Five deaths were associated with an influenza B virus and occurred during weeks 1, 3, and 4 (the week ending January 6, January 20, and January 27, 2018, respectively).
A total of 53 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
One death that occurred during the 2015-2016 season was associated with an influenza A virus for which no subtyping was performed and occurred during week 28 (the week ending July 16, 2016). This death brings the total number of reported influenza-associated deaths occurring during that season to 93.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
A total of 14,676 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and January 27, 2018. The overall hospitalization rate was 51.4 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (226.8 per 100,000 population), followed by adults aged 50-64 (54.0 per 100,000 population) and children aged 0-4 years (33.3 per 100,000 population). Among 14,676 hospitalizations, 12,849 (87.5%) were associated with influenza A virus, 1,762 (12.0%) with influenza B virus, 35 (0.2%) with influenza A virus and influenza B virus co-infection, and 30 (0.2%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 2,797 (86.5%) were A(H3N2) and 437 (13.5%) were A(H1N1)pdm09 virus.
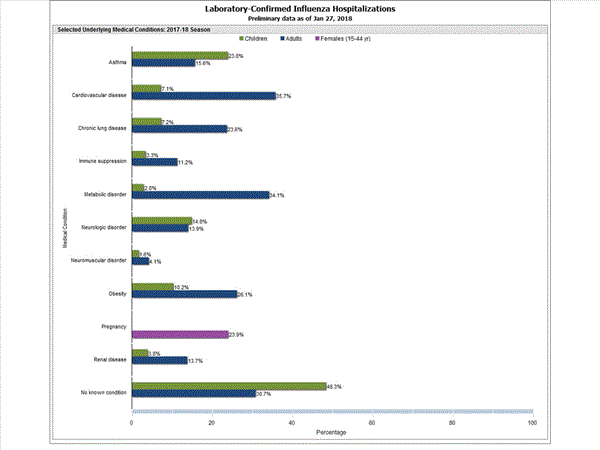
Among 1,708 hospitalized adults with information on underlying medical conditions, 1,183 (69.3%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 180 hospitalized children with information on underlying medical conditions, 93 (51.7%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 138 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 33 (23.9%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Outpatient Illness Surveillance:
Nationwide during week 4, 7.1% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%.(ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
On a regional level, the percentage of outpatient visits for ILI ranged from 2.8% to 13.0% during week 4. All 10 regions reported percentages of outpatient visits for ILI at or above their region specific baselines.
A midseason glimpse of flu vaccine effectiveness (VE) in Canada shows that protection against the H3N2 strain is very low, similar to what Australian scientists reported in the fall
Friday, February 2nd, 2018“….As reported from Australia for the 2017 southern hemisphere vaccine, interim estimates from Canada for the 2017/18 northern hemisphere vaccine indicate low VE of less than 20% against influenza A(H3N2), notably among working-age adults. While the influenza A(H3N2) epidemic continues, adjunct protective measures should be reinforced to minimise the associated disease burden in high-risk individuals….”
Democratic People’s Republic of Korea (DPRK) Red Cross Society & The Arrival of H1N1 in North Korea
Tuesday, January 30th, 2018“On 19 January 2018 the Vice Minister of Public Health (MoPH) officially informed the World Health Organization (WHO) Country Office in Pyongyang of an outbreak of Influenza A (H1N1) stating that between 1 December 2017 and 16 January 2018 there was a total of 126,574 suspected influenza cases – individuals presenting with influenza-like illness. Of these, 81,640 cases were confirmed as Influenza A (H1N1) and as per the Ministry communication there had been four deaths – three children and one adult. According to the Ministry, 24.5 per cent of suspected influenza cases (numbering 31,010) were among children aged 0-7 years, 22.8 per cent (n= 28,858) were among children 8-16 years and the rest 52.7 per cent (n= 66,706), were among those who were above 17 years. The outbreak has become generalized throughout the country with 28.7 per cent of cases in the capital city – Pyongyang. The government has requested support for influenza vaccination targeting high-risk individuals with the MoPH specifically requesting 30,000 Oseltamivir tablets for healthcare workers. WHO has so far dispatched 5,000 tablets with 30,000 in the pipeline to distribute to frontline healthcare workers and vulnerable groups. There is a request to strengthen the nonpharmaceutical aspect of the operation with an emphasis on public health including surveillance and preventive activities with all agencies (WHO, UNICEF) requested to support with conducting an effective communication programme…..”
2017-2018 Influenza Season Week 3 ending January 20, 2018: Influenza activity increased in the United States.
Friday, January 26th, 2018Synopsis:
During week 3 (January 14-20, 2018), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 3 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories slightly increased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Seven influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 41.9 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 6.6%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, Puerto Rico, and 39 states experienced high ILI activity; the District of Columbia and five states experienced moderate ILI activity; three states experienced low ILI activity; and three states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread; Guam reported regional activity; the District of Columbia and one state reported local activity; and the U.S. Virgin Islands reported sporadic activity.
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories, which include both public health and clinical laboratories located in all 50 states, Puerto Rico, and the District of Columbia, report to CDC the total number of respiratory specimens tested for influenza and the number positive for influenza by virus type. In addition, public health laboratories also report the influenza A subtype (H1 or H3) and influenza B lineage information of the viruses they test and the age or age group of the persons from whom the specimens were collected.
Additional virologic data, including national, regional and select state-level data, can be found at: http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html. Age group proportions and totals by influenza subtype reported by public health laboratories can be found at: http://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
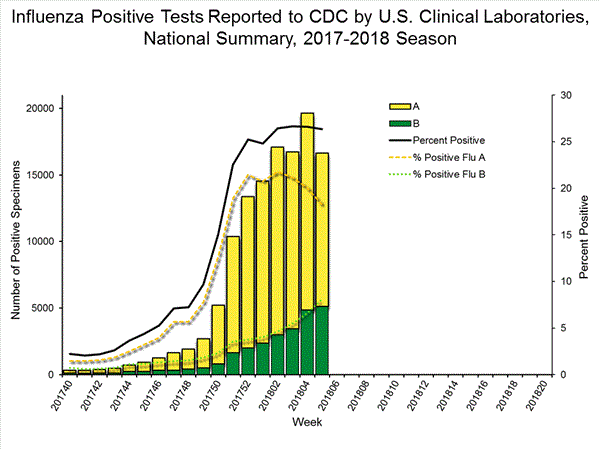
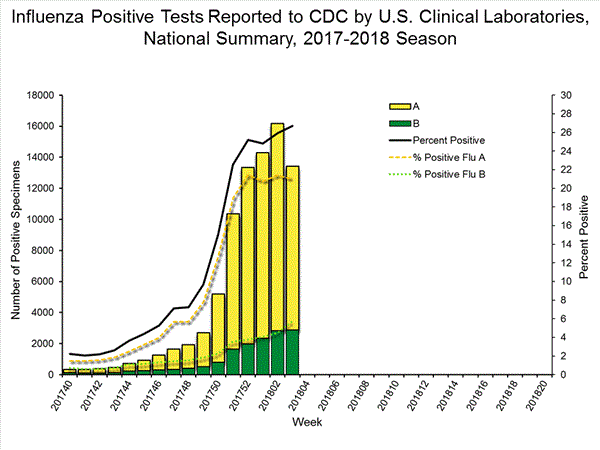
The results of tests performed by clinical laboratories are summarized below.
| Week 3 | Data Cumulative since October 1, 2017 (Week 40) |
|
|---|---|---|
| No. of specimens tested | 50,276 | 513,252 |
| No. of positive specimens (%) | 13,421 (26.7%) | 83,450 (16.3%) |
| Positive specimens by type | ||
| Influenza A | 10,536 (78.5%) | 68,517 (82.1%) |
| Influenza B | 2,885 (21.5%) | 14,933 (17.9%) |
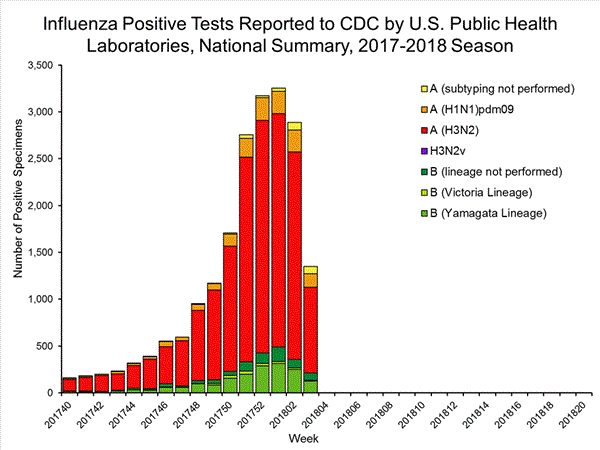
The results of tests performed by public health laboratories, as well as the age group distribution of influenza positive tests, during the current week are summarized below.
| Week 3 | Data Cumulative since October 1, 2017 (Week 40) |
|
|---|---|---|
| No. of specimens tested | 2,209 | 39,400 |
| No. of positive specimens* | 1,349 | 19,869 |
| Positive specimens by type/subtype | ||
| Influenza A | 1,136 (84.2%) | 17,205 (86.6%) |
| A(H1N1)pmd09 | 144 (12.7%) | 1,530 (8.9%) |
| H3N2 | 914 (80.5%) | 15,376 (89.4%) |
| Subtyping not performed | 78 (6.9%) | 299 (1.7%) |
| Influenza B | 213 (15.8%) | 2,664 (13.2%) |
| Yamagata lineage | 127 (59.6%) | 1,750 (65.7%) |
| Victoria lineage | 8 (3.8%) | 184 (6.9%) |
| Lineage not performed | 78 (36.6%) | 730 (27.4%) |
*The percent of specimens testing positive for influenza is not reported because public health laboratories often receive samples that have already tested positive for influenza at a clinical laboratory and therefore percent positive would not be a valid indicator of influenza activity. Additional information is available at http://www.cdc.gov/flu/weekly/overview.htm.
Influenza Virus Characterization:
Close monitoring of influenza viruses is required to better assess the potential impact on public health. CDC characterizes influenza viruses through one or more tests including genomic sequencing and hemagglutination inhibition (HI) (i.e., hemagglutination inhibition (HI) and/or neutralization assays). These data are used to monitor for changes in circulating influenza viruses and to compare how similar currently circulating influenza viruses are to the reference viruses used for developing influenza vaccines. Antigenic and genetic characterization of circulating influenza viruses can give an indication of the influenza vaccine’s ability to produce an immune response against the wide array of influenza viruses co-circulating, but annual vaccine effectiveness estimates are needed to determine how much protection has been provided to the population by vaccination.
For nearly all influenza-positive surveillance samples received at CDC, next-generation sequencing is performed to determine the genetic identity of circulating influenza viruses and to monitor viruses for evidence of genetic changes. Viruses are classified into genetic clades/subclades based on analysis of the genetic sequences of the HA gene segments. However, genetic changes do not always result in antigenic change. Extensive genetic variation may exist in circulating viruses, with no evidence of substantial antigenic drift. Antigenic drift is evaluated by comparing cell-propagated circulating viruses with cell-propagated reference viruses representing currently recommended vaccine components.
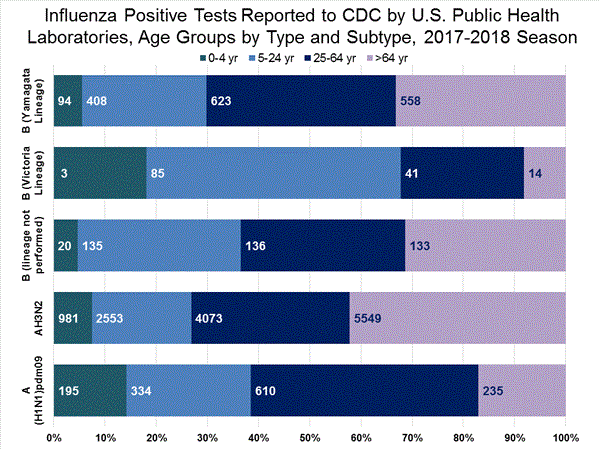
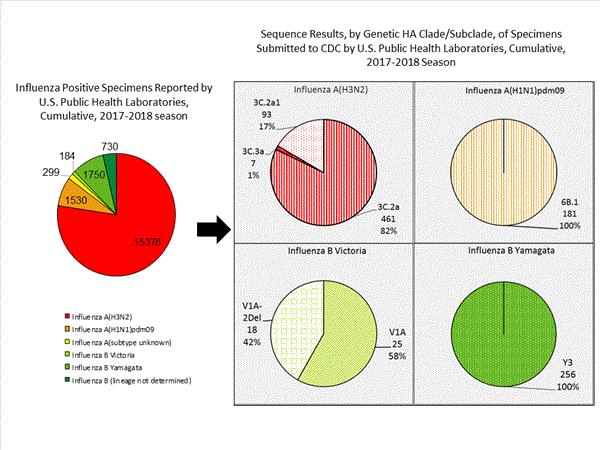
CDC has antigenically or genetically characterized 1,041 influenza viruses collected during October 1, 2017 – January 20, 2018, and submitted by U.S. laboratories, including 181 influenza A(H1N1)pdm09 viruses, 561 influenza A(H3N2) viruses, and 299 influenza B viruses.
- A (H1N1)pdm09: Phylogenetic analysis of the HA genes from 181 A(H1N1)pdm09 viruses showed that all belonged to clade 6B.1. Eighty-five A(H1N1)pdm09 viruses were antigenically characterized, and all were antigenically similar (analyzed using HI with ferret antisera) to the reference 6B.1 virus A/Michigan/45/2015, representing the recommended influenza A(H1N1)pdm09 reference virus for the 2017–18 Northern Hemisphere influenza vaccines.
- A (H3N2): Phylogenetic analysis of the HA genes from 561 A(H3N2) viruses revealed extensive genetic diversity with multiple clades/subclades co-circulating. The HA genes of circulating viruses belonged to clade 3C.2a (n=461), subclade 3C.2a1 (n=93) or clade 3C.3a (n=7). One hundred ninety four influenza A(H3N2) viruses were antigenically characterized, and 191 (98.5%) A(H3N2) viruses tested were well-inhibited (reacting at titers that were within fourfold of the homologous virus titer) by ferret antisera raised against A/Michigan/15/2014 (3C.2a), a cell propagated A/Hong Kong/4801/2014-like reference virus representing the A(H3N2) component of 2017–18 Northern Hemisphere influenza vaccines.
Influenza B Viruses
- B/Victoria: Phylogenetic analysis of 43 B/Victoria-lineage viruses indicate that all HA genes belonged to genetic clade V1A, the same genetic clade as the vaccine reference virus, B/Brisbane/60/2008. However, a small number of viruses had a 6-nucleotide deletion (encoding amino acids 162 and 163) in the HA (abbreviated as V1A-2Del). Sixteen (59.3%) B/Victoria lineage viruses were well-inhibited by ferret antisera raised against cell -propagated B/Brisbane/60/2008 reference virus, representing a recommended B virus component of 2017–18 Northern Hemisphere influenza vaccines. Eleven (40.7%) B/Victoria lineage viruses reacted poorly (at titers that were 8-fold or greater reduced compared with the homologous virus titer) with ferret antisera raised against cell-propagated B/Brisbane/60/2008, and these viruses had the V1A-2Del HA.
- B/Yamagata: Phylogenetic analysis of 256 influenza B/Yamagata-lineage viruses indicate that the HA genes belonged to clade Y3. A total of 152 influenza B/Yamagata-lineage viruses were antigenically characterized, and all were antigenically similar to cell propagated B/Phuket/3073/2013, the reference vaccine virus representing the influenza B/Yamagata-lineage component of the 2017–18 Northern Hemisphere quadrivalent vaccines.
The majority of U.S. viruses submitted for characterization come from state and local public health laboratories. Due to Right Size Roadmap considerations, specimen submission guidance to laboratories is that, if available, 2 influenza A(H1N1)pdm09, 2 influenza A(H3N2), and 2 influenza B viruses be submitted every other week.. Therefore, the numbers of each virus type/subtype characterized should be more balanced across subtypes/lineages but will not reflect the actual proportion of circulating viruses. In the figure below, the results of tests performed by public health labs are shown on the left and CDC sequence results (by genetic clade/subclade) are shown on the right.
Antiviral Resistance:
Testing of influenza A (H1N1)pdm09, influenza A (H3N2), and influenza B virus isolates for resistance to neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) is performed at CDC using a functional assay. Additional influenza A (H1N1)pdm09 and influenza A (H3N2) viruses from clinical samples are tested for mutations known to confer oseltamivir resistance. The data summarized below combine the results of both testing methods. These samples are routinely obtained for surveillance purposes rather than for diagnostic testing of patients suspected to be infected with antiviral-resistant virus.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A (H1N1)pdm09 and influenza A (H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, data from adamantane resistance testing are not presented below.
|
Oseltamivir |
Zanamivir |
Peramivir |
||||
|---|---|---|---|---|---|---|
|
Virus Samples tested (n) |
Resistant Viruses, Number (%) |
Virus Samples tested (n) |
Resistant Viruses, Number (%) |
Virus Samples tested (n) |
Resistant Viruses, Number (%) |
|
| Influenza A (H1N1)pdm09 |
181 |
2 (1.1) |
147 |
0 (0.0) |
181 |
2 (1.1) |
| Influenza A (H3N2) |
645 |
0 (0.0) |
645 |
0 (0.0) |
474 |
0 (0.0) |
| Influenza B |
229 |
0 (0.0) |
229 |
0 (0.0) |
229 |
0 (0.0) |
On December 27, 2017, a Health Advisory was released by CDC providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment. More information is available at https://emergency.cdc.gov/han/han00409.asp.
The majority of recently circulating influenza viruses are susceptible to the neuraminidase inhibitor antiviral medications, oseltamivir, zanamivir, and peramivir; however, rare sporadic instances of oseltamivir-resistant and peramivir-resistant influenza A(H1N1)pdm09 viruses and oseltamivir-resistant influenza A(H3N2) viruses have been detected worldwide. Antiviral treatment as early as possible is recommended for patients with confirmed or suspected influenza who have severe, complicated, or progressive illness; who require hospitalization; or who are at high risk for serious influenza-related complications. Additional information on recommendations for treatment and chemoprophylaxis of influenza virus infection with antiviral agents is available at http://www.cdc.gov/flu/antivirals/index.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance:
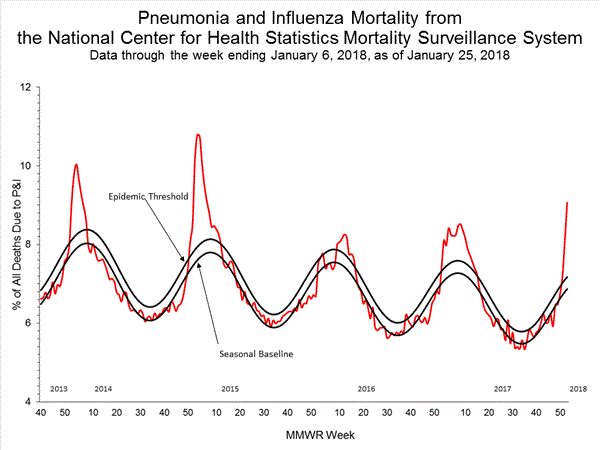
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on January 25, 2018, 9.1% of the deaths occurring during the week ending January 6, 2018 (week 1) were due to P&I. This percentage is above the epidemic threshold of 7.2% for week 1.
Background: Weekly mortality surveillance data include a combination of machine coded and manually coded causes of death collected from death certificates. Percentages of deaths due to P&I are higher among manually coded records than more rapidly available machine coded records. Due to the additional time needed for manual coding, the initially reported P&I percentages may be lower than percentages calculated from final data. Previous longer backlogs in manual coding have been resolved and death records are now coded within 10 days from receipt of a death record by NCHS.
Region and state-specific data are available at http://gis.cdc.gov/grasp/fluview/mortality.html.
Influenza-Associated Pediatric Mortality:
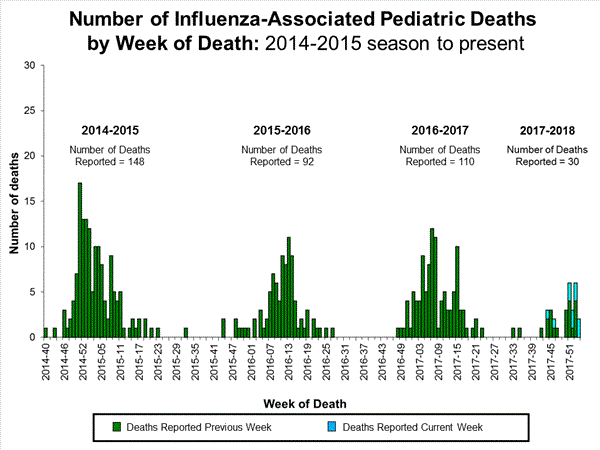
Seven influenza-associated pediatric deaths were reported to CDC during week 3. One death was associated with an influenza A(H3) virus and occurred during week 2 (the week ending January 13, 2018). Two deaths were associated with an influenza A(H1N1)pdm09 virus and occurred during weeks 1 and 3 (the weeks ending January 6, 2018, and January 20, 2018, respectively). Three deaths were associated with an influenza A virus for which no subtyping was performed and occurred during weeks 52 and 1 (the weeks ending December 30, 2017, and January 6, 2018, respectively). One death was associated with an influenza B virus and occurred during week 2.
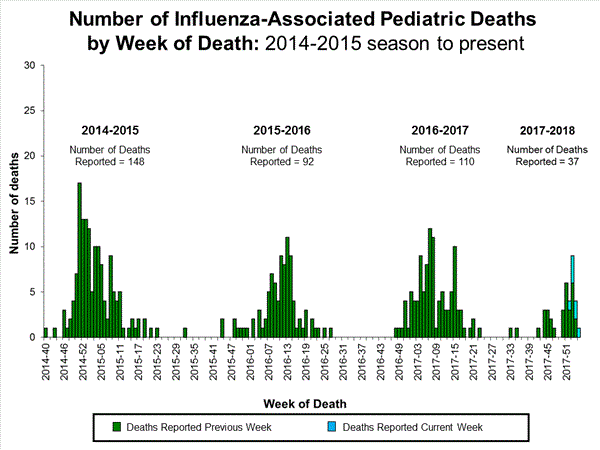
A total of 37 influenza-associated pediatric deaths have been reported for the 2017-2018 season.
Additional data can be found at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
Influenza-Associated Hospitalizations:
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in children younger than 18 years of age (since the 2003-2004 influenza season) and adults (since the 2005-2006 influenza season).
The FluSurv-NET covers more than 70 counties in the 10 Emerging Infections Program (EIP) states (CA, CO, CT, GA, MD, MN, NM, NY, OR, and TN) and additional Influenza Hospitalization Surveillance Project (IHSP) states. The IHSP began during the 2009-2010 season to enhance surveillance during the 2009 H1N1 pandemic. IHSP sites included IA, ID, MI, OK and SD during the 2009-2010 season; ID, MI, OH, OK, RI, and UT during the 2010-2011 season; MI, OH, RI, and UT during the 2011-2012 season; IA, MI, OH, RI, and UT during the 2012-2013 season; and MI, OH, and UT during the 2013-2014, 2014-15, 2015-16, 2016-17, and 2017-18 seasons.
Data gathered are used to estimate age-specific hospitalization rates on a weekly basis, and describe characteristics of persons hospitalized with influenza illness. The rates provided are likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications.
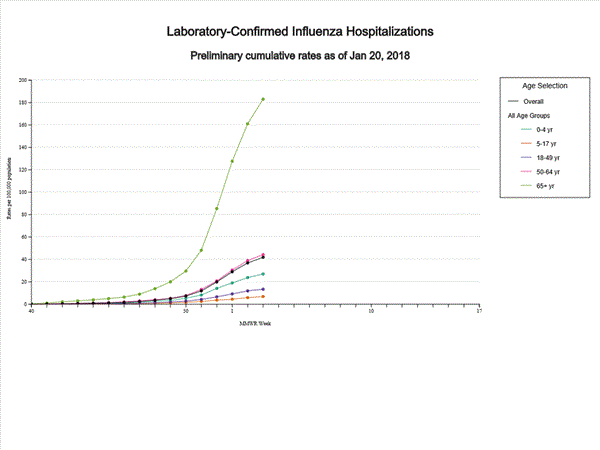
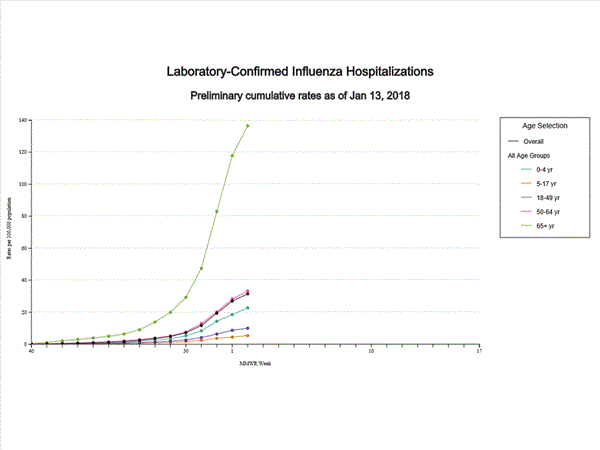
A total of 11,965 laboratory-confirmed influenza-associated hospitalizations were reported between October 1, 2017 and January 20, 2018. The overall hospitalization rate was 41.9 per 100,000 population. The highest rate of hospitalization was among adults aged ≥65 years (183.1 per 100,000 population), followed by adults aged 50-64 (44.2 per 100,000 population) and children aged 0-4 years (27.0 per 100,000 population). Among 11,965 hospitalizations, 10,612 (88.7%) were associated with influenza A virus, 1,295 (10.8%) with influenza B virus, 28 (0.2%) with influenza A virus and influenza B virus co-infection, and 30 (0.3%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 2,360 (86.4%) were A(H3N2) and 372 (13.6%) were A(H1N1)pdm09 virus.
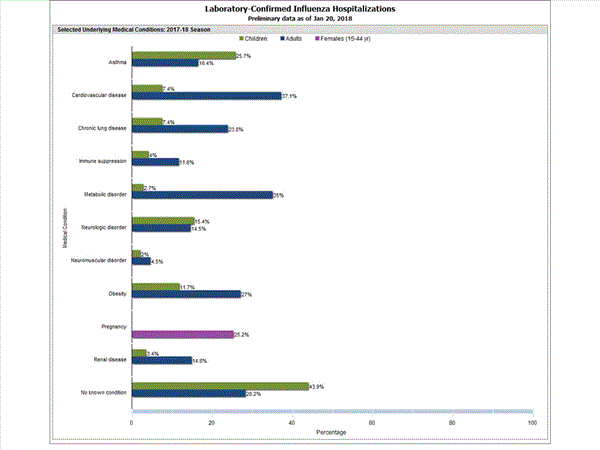
Among 1,445 hospitalized adults with information on underlying medical conditions, 1,038 (71.8%) had at least one reported underlying medical condition; the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 148 hospitalized children with information on underlying medical conditions, 83 (56.1%) had at least one underlying medical condition; the most commonly reported were asthma, neurologic disorder, and obesity. Among 115 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 29 (25.2%) were pregnant.
Additional FluSurv-NET data can be found at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html and http://gis.cdc.gov/grasp/fluview/FluHospChars.html.
Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET), a population-based surveillance for influenza related hospitalizations in children and adults in 13 U.S. states. Cumulative incidence rates are calculated using the National Center for Health Statistics’ (NCHS) population estimates for the counties included in the surveillance catchment area.
FluSurv-NET data are preliminary and displayed as they become available. Therefore, figures are based on varying denominators as some variables represent information that may require more time to be collected. Data are refreshed and updated weekly. Asthma includes a medical diagnosis of asthma or reactive airway disease; Cardiovascular diseases include conditions such as coronary heart disease, cardiac valve disorders, congestive heart failure, and pulmonary hypertension; does not include isolated hypertension; Chronic lung diseases include conditions such as chronic obstructive pulmonary disease, bronchiolitis obliterans, chronic aspiration pneumonia, and interstitial lung disease; Immune suppression includes conditions such as immunoglobulin deficiency, leukemia, lymphoma, HIV/AIDS, and individuals taking immunosuppressive medications; Metabolic disorders include conditions such as diabetes mellitus; Neurologic diseases include conditions such as seizure disorders, cerebral palsy, and cognitive dysfunction; Neuromuscular diseases include conditions such as multiple sclerosis and muscular dystrophy; Obesity was assigned if indicated in patient’s medical chart or if body mass index (BMI) >30 kg/m2; Pregnancy percentage calculated using number of female cases aged between 15 and 44 years of age as the denominator; Renal diseases include conditions such as acute or chronic renal failure, nephrotic syndrome, glomerulonephritis, and impaired creatinine clearance; No known condition indicates that the case did not have any known high risk medical condition indicated in medical chart at the time of hospitalization.
Outpatient Illness Surveillance:
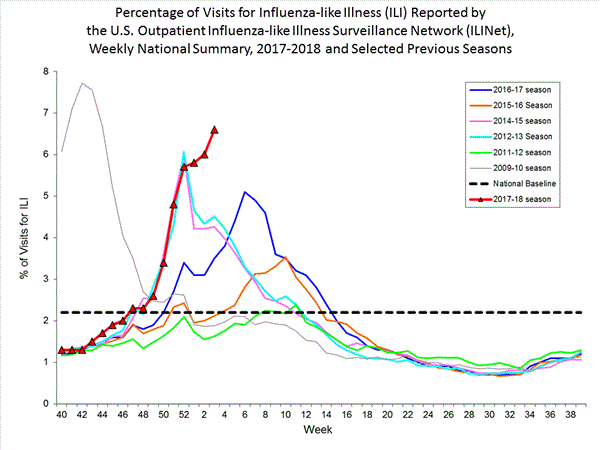
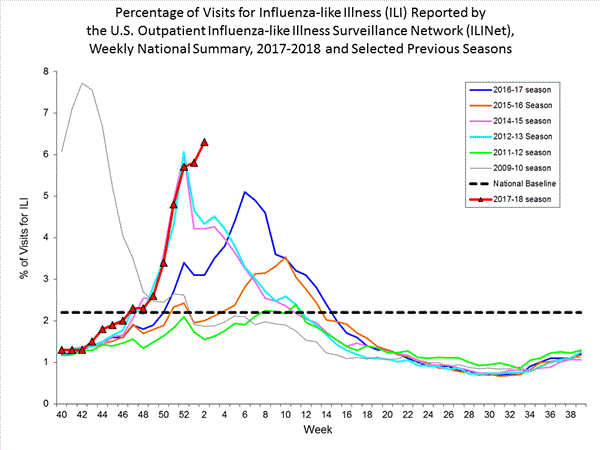
Nationwide during week 3, 6.6% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.2%.(ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.)
Additional ILINet data, including national, regional and select state-level data, are available at http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
On a regional level, the percentage of outpatient visits for ILI ranged from 2.9% to 11.7% during week 3. All 10 regions reported percentages of outpatient visits for ILI at or above their region specific baselines.
What is Interferon and what does it have to do with the flu?
Monday, January 22nd, 2018Interferon “….(IFN) expression is a mammal’s first response to viral infection. Many viruses have thus evolved mechanisms to evade IFN. Du et al. developed a method to systematically ablate IFN evasion genes from live, attenuated influenza virus…. A combination of mutants was assembled to construct a virus that triggered transient IFN responses in mice but that was unable to replicate effectively. The transient IFN responses led to robust antibody and memory responses that protected against subsequent challenge with different influenza viruses. This approach could be adapted to improve other RNA virus vaccines.
On the way to creating a ‘universal’ flu vaccine
Monday, January 22nd, 2018‘…..The key to the new vaccine is an understanding of the interactions between the virus and interferons, which are proteins that are critical to the body’s immune response. Interferons have two main functions: one is a first line of defense to kill invading viruses very quickly; a second is to coordinate the adaptive immune responses, which provide long-lasting protection against the virus. The latter is the basis of vaccination.
“If viruses do not induce interferons, they will not be killed in the first-line defense; and without interferons, the adaptive immune response is limited,” said Sun, who also is a professor of bioengineering at the UCLA Henry Samueli School of Engineering and Applied Science. “For these reasons, viruses have evolved strategies to evade detection and limit the production of interferons by host organisms.”……’
New research shows that the flu virus can be airborne not just carried by droplets
Saturday, January 20th, 2018Osterholm: “Respiratory droplets are like boulders that fall quickly over a short distance, while aerosols are like the perfume that can be smelled from a store’s fragrance department three aisles away.”
and Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community PNAS 2018 ; published ahead of print January 18, 2018, doi:10.1073/pnas.1716561115
Research significance: “…..We show that sneezing is rare and not important for—and that coughing is not required for—influenza virus aerosolization….”
During week 2 (January 7-13, 2018), influenza activity increased in the United States.
Friday, January 19th, 2018During week 2 (January 7-13, 2018), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 2 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was above the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Ten influenza-associated pediatric deaths were reported
- Influenza-associated Hospitalizations: A cumulative rate of 31.5 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 6.3%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City, Puerto Rico, and 32 states experienced high ILI activity; 9 states experienced moderate ILI activity; the District of Columbia and six states experienced low ILI activity; and three states experienced minimal ILI activity.
- Geographic Spread of Influenza: The geographic spread of influenza in Puerto Rico and 49 states was reported as widespread; Guam reported regional activity; the District of Columbia and one state reported local activity; and the U.S. Virgin Islands reported sporadic activity.
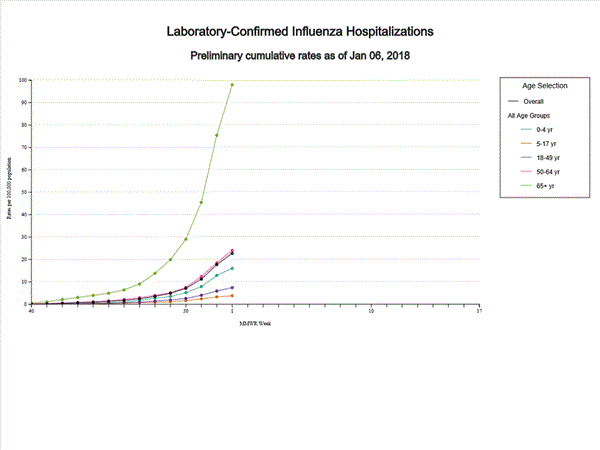
2017-2018 Influenza Season Week 1 ending January 6, 2018
Sunday, January 14th, 2018During week 1 (December 31, 2017-January 6, 2018), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 1 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories remained elevated.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was at the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Seven influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 22.7 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 5.8%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City and 26 states experienced high ILI activity; Puerto Rico and 10 states experienced moderate ILI activity; the District of Columbia and six states experienced low ILI activity; and eight states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in 49 states was reported as widespread; Guam and one state reported regional activity; the District of Columbia reported local activity; the U.S. Virgin Islands reported sporadic activity; and Puerto Rico did not report.