Archive for the ‘Influenza’ Category
2017-2018 Influenza Season Week 52 ending December 30, 2017
Wednesday, January 10th, 2018Synopsis:
During week 52 (December 24-30, 2017), influenza activity increased sharply in the United States.
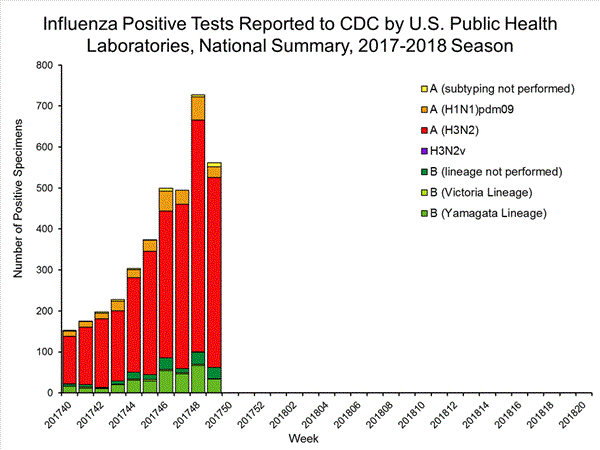
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 52 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
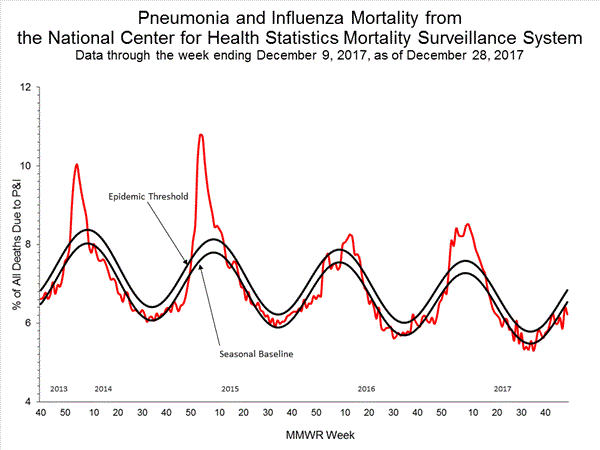
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
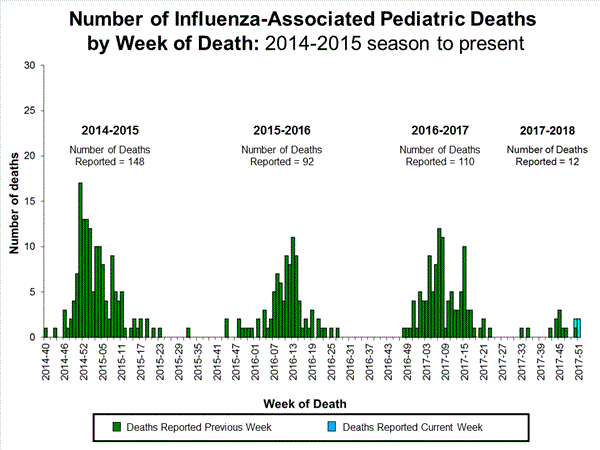
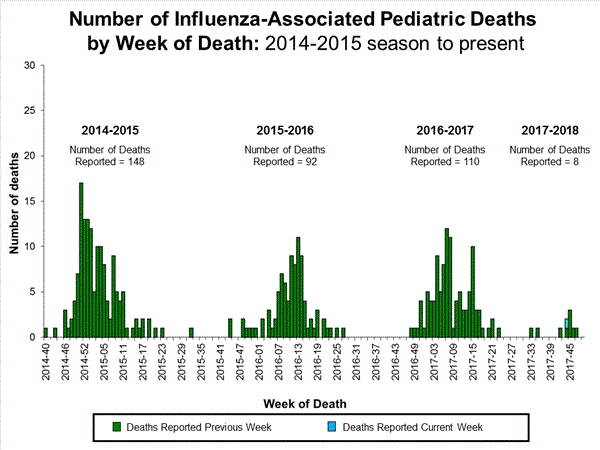
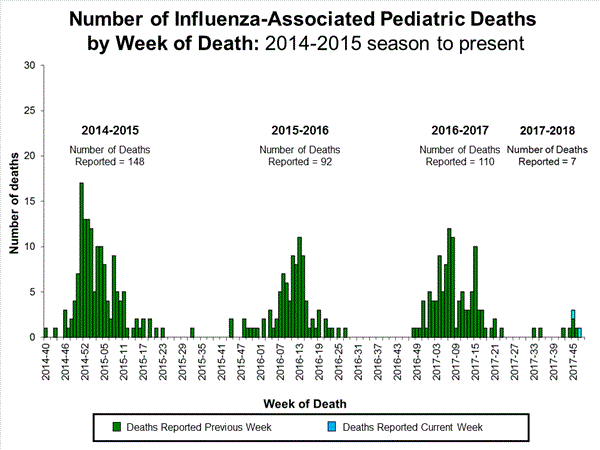
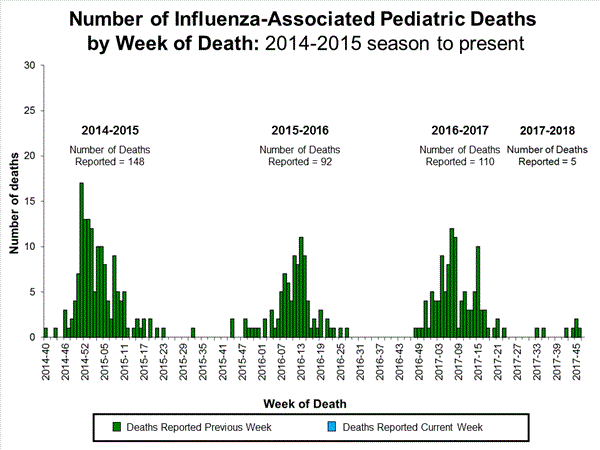
- Influenza-associated Pediatric Deaths: One influenza-associated pediatric death was reported.
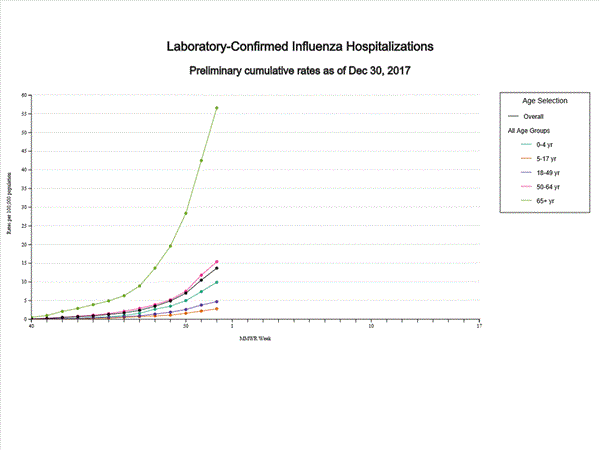
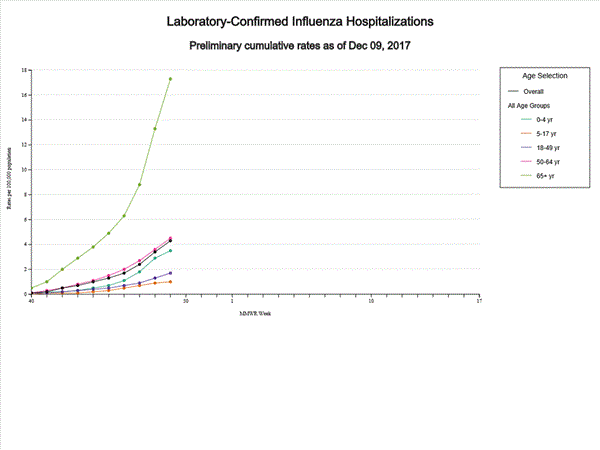
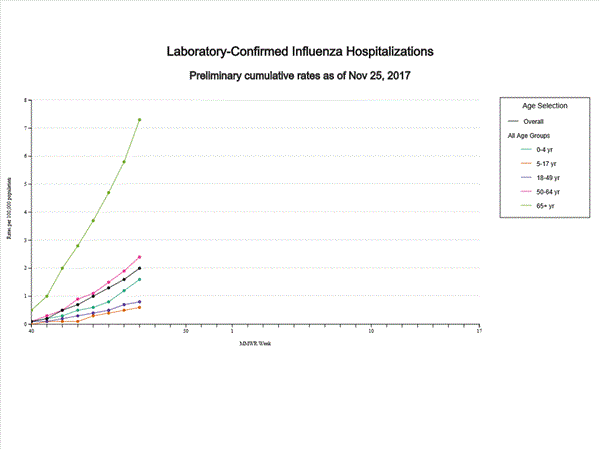
- Influenza-associated Hospitalizations: A cumulative rate of 13.7 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
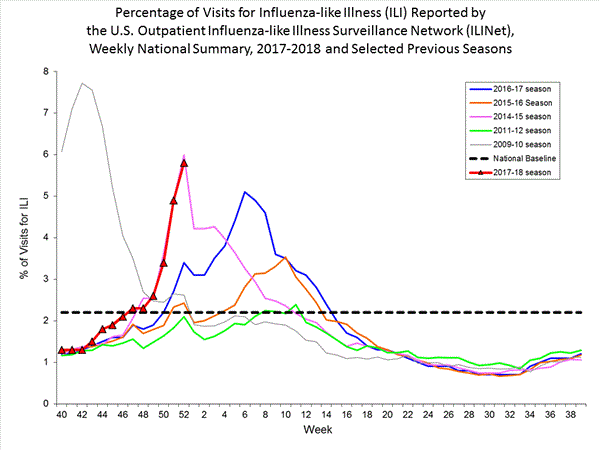
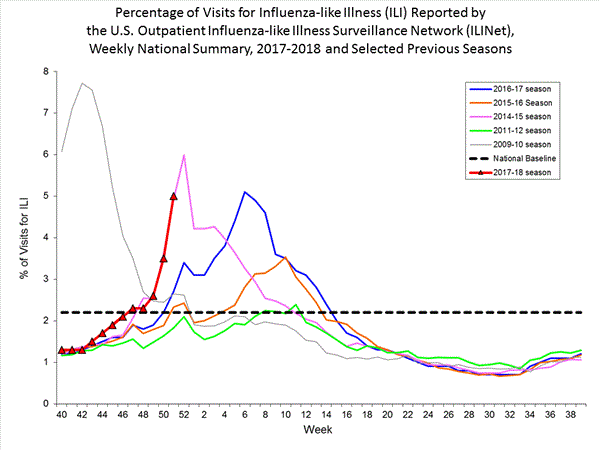
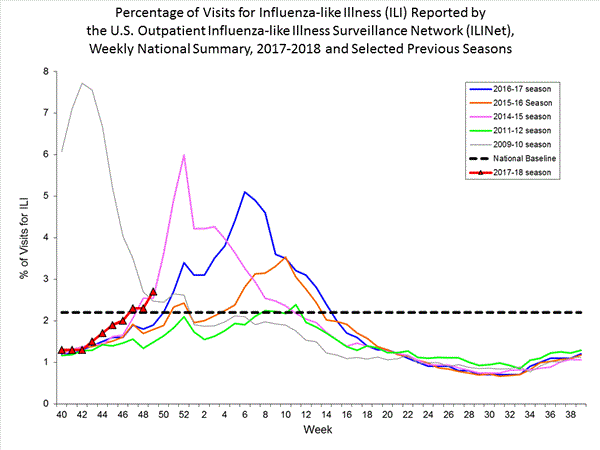
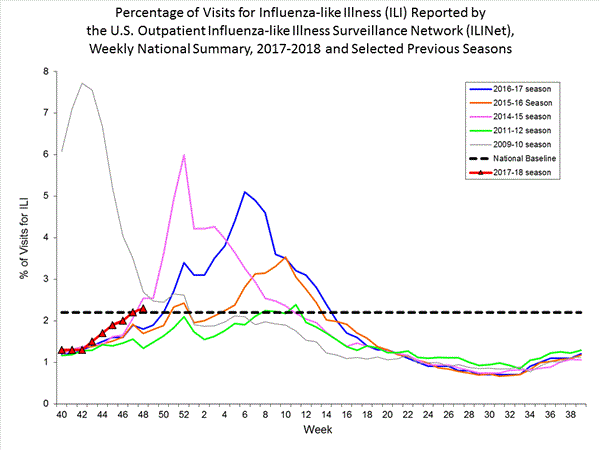
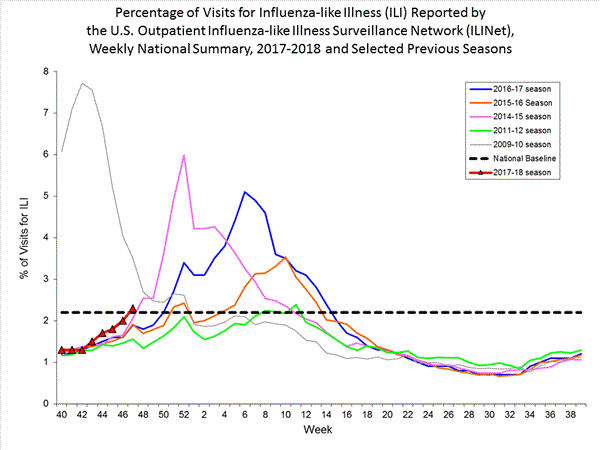
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 5.8%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. New York City and 26 states experienced high ILI activity; Puerto Rico and nine states experienced moderate ILI activity; the District of Columbia and six states experienced low ILI activity; and nine states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in 46 states was reported as widespread; four states reported regional activity; the District of Columbia reported local activity; and Guam, Puerto Rico, and the U.S. Virgin Islands did not report.
CDC: About increased influenza A(H3N2) activity
Sunday, December 31st, 2017Seasonal Influenza A(H3N2) Activity and Antiviral Treatment of Patients with Influenza
Distributed via the CDC Health Alert Network
December 27, 2017, 1030 ET (10:30 AM ET)
CDCHAN-00409
Summary
The Centers for Disease Control and Prevention (CDC) is providing: 1) a notice about increased influenza A(H3N2) activity and its clinical implications; 2) a summary of influenza antiviral drug treatment recommendations; 3) an update about approved treatment drugs and supply this season; and 4) background information for patients about influenza treatment.
Background
In the United States (U.S.), influenza activity has increased significantly over recent weeks with influenza A(H3N2) viruses predominating so far this season. In the past, A(H3N2) virus-predominant influenza seasons have been associated with more hospitalizations and deaths in persons aged 65 years and older and young children compared to other age groups. In addition, influenza vaccine effectiveness (VE) in general has been lower against A(H3N2) viruses than against influenza A(H1N1)pdm09 or influenza B viruses. Last season, VE against circulating influenza A(H3N2) viruses was estimated to be 32% in the U.S. CDC expects that VE could be similar this season, should the same A(H3N2) viruses continue to predominate. For this reason, in addition to influenza vaccination for prevention of influenza, the use of antiviral medications for treatment of influenza becomes even more important than usual. The neuraminidase inhibitor (NAI) antiviral medications are most effective in treating influenza and reducing complications when treatment is started early. Evidence from previous influenza seasons suggests that NAI antivirals are underutilized in outpatients and hospitalized patients with influenza who are recommended for treatment.
This CDC Health Advisory is being issued to—
- Remind clinicians that influenza should be high on their list of possible diagnoses for ill patients because influenza activity is increasing nationwide, and
- Advise clinicians that all hospitalized patients and all high-risk patients (either hospitalized or outpatient) with suspected influenza should be treated as soon as possible with a neuraminidase inhibitor antiviral. While antiviral drugs work best when treatment is started within 2 days of illness onset, clinical benefit has been observed even when treatment is initiated later.
Recommendations
1. CDC Antiviral Recommendations for the 2017–2018 Season
CDC recommends antiviral medications for treatment of influenza as an important adjunct to annual influenza vaccination. Treatment with neuraminidase inhibitors has been shown to have clinical and public health benefit in reducing illness and severe outcomes of influenza based on evidence from randomized controlled trials, meta-analyses of randomized controlled trials, and observational studies during past influenza seasons and during the 2009 H1N1 pandemic.1,2,3,4,5,6
2. All Hospitalized, Severely Ill, and High-Risk Patients with Suspected or Confirmed Influenza Should Be Treated with Antivirals
Any patient with suspected or confirmed influenza in the following categories should be treated as soon as possible with a neuraminidase inhibitor:
1) Any patient who is hospitalized—treatment is recommended for all hospitalized patients;
2) Any patient who has severe, complicated, or progressive illness—this may include outpatients with severe or prolonged progressive symptoms or who develop complications such as pneumonia but who are not hospitalized;
3) Any patient who is at higher risk for influenza complications but not hospitalized. Patients in this group include—
- children younger than 2 years (although all children younger than 5 years are considered at higher risk for complications from influenza, the highest risk is for those younger than 2 years)
- adults aged 65 years and older
- persons with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematological (including sickle cell disease), and metabolic disorders (including diabetes mellitus), or neurologic and neurodevelopment conditions (including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy [seizure disorders], stroke, intellectual disability [mental retardation], moderate to severe developmental delay, muscular dystrophy, or spinal cord injury)
- people with immunosuppression, including that caused by medications or by HIV infection
- women who are pregnant or postpartum (within 2 weeks after delivery)
- people aged younger than 19 years who are receiving long-term aspirin therapy
- American Indians/Alaska Natives
- people with extreme obesity (i.e., body-mass index is equal to or greater than 40)
- residents of nursing homes and other chronic-care facilities
3. Timing of Treatment and Implications for Patient Evaluation, Treatment, and Testing
Clinical benefit is greatest when antiviral treatment is administered as early as possible after illness onset. Therefore, antiviral treatment should be started as soon as possible after illness onset and should not be delayed even for a few hours to wait for the results of testing. Ideally, treatment should be initiated within 48 hours of symptom onset. However, antiviral treatment initiated later than 48 hours after illness onset can still be beneficial for some patients.
A very large observational study of more than 29,000 hospitalized influenza patients reported that while the greatest clinical benefit was found when antiviral treatment was initiated within 48 hours of illness onset, starting antiviral treatment more than 2 days after onset had survival benefit in adults versus no treatment. 6 Also, a randomized, placebo-controlled study suggested clinical benefit when oseltamivir was initiated 72 hours after illness onset among febrile children with uncomplicated influenza.7 Clinical judgment, on the basis of the patient’s disease severity and progression, age, underlying medical conditions, likelihood of influenza, and time since onset of symptoms, is important when making antiviral treatment decisions for outpatients, particularly those who are not at increased risk for influenza complications.
Because of the importance of early treatment, decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza. Therefore, empiric antiviral treatment should generally be initiated as soon as possible when there is known influenza activity in the community. A history of current season influenza vaccination does not exclude a diagnosis of influenza in an ill child or adult. During influenza season especially, high-risk patients should be advised to call their provider promptly if they have symptoms of influenza. It may be useful for providers to implement phone triage lines to enable high-risk patients to discuss symptoms over the phone. To facilitate early initiation of treatment, when feasible, an antiviral prescription can be provided without testing and before an office visit.
4. Influenza Testing
Information to assist clinicians about influenza testing decisions is available at https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm. The most accurate influenza tests are molecular assays. Rapid molecular assays are available in clinical settings that can detect influenza virus nucleic acids in respiratory specimens in 15-30 minutes with high sensitivity and specificity. Other approved molecular assays can yield results in 60-80 minutes or in several hours with very high sensitivity and specificity.
For hospitalized patients with suspected influenza, molecular assays are recommended. Information on influenza molecular assays is available at https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm. Rapid influenza diagnostic tests (RIDTs) with an analyzer device can detect influenza A and B viral nucleoprotein antigens in respiratory specimens in 10-15 minutes with moderate sensitivity, and RIDTs without an analyzer device have low to moderate sensitivity compared with reverse transcription-polymerase chain reaction (RT-PCR).
Proper interpretation of influenza testing results is important to guide optimal management of influenza patients. An algorithm to assist clinicians in interpreting the results of influenza testing when influenza viruses ARE circulating in the community is available at https://www.cdc.gov/flu/professionals/diagnosis/algorithm-results-circulating.htm. Clinicians should be aware that a negative RIDT result does not exclude a diagnosis of influenza in a patient with suspected influenza when there is influenza activity in the community. Other factors such as the quality of the specimen, the source of the specimen in the respiratory tract, and the timing of specimen collection in relationship to illness onset, may also affect test results.
5. Antivirals in Non-High Risk Patients with Uncomplicated Influenza
Neuraminidase inhibitors can benefit other individuals with influenza. While current guidance focuses on antiviral treatment of those with severe illness or at high risk of complications from influenza, antiviral treatment may be prescribed on the basis of clinical judgment for any previously healthy (non-high risk) outpatient with suspected or confirmed influenza who presents within 2 days after illness onset. Neuraminidase inhibitors can reduce the duration of uncomplicated influenza illness by approximately 1 day when started within 2 days after illness onset in otherwise healthy persons. It is possible that antiviral treatment started after 48 hours may offer some benefit. 7
6. Antiviral Medications
Three prescription neuraminidase inhibitor antiviral medications are approved by the U.S. Food and Drug
Administration (FDA) and are recommended for use in the U.S. during the 2017–2018 influenza season: oseltamivir (available as a generic version or under the trade name Tamiflu®), zanamivir (Relenza®), and peramivir (Rapivab®).
- Oral oseltamivir is FDA-approved for treatment of uncomplicated influenza within 2 days of illness onset in persons aged 2 weeks and older, and for chemoprophylaxis to prevent influenza in people 1 year of age and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants younger than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year of age, is recommended by CDC and the American Academy of Pediatrics. Due to limited data, use of oseltamivir for chemoprophylaxis is not recommended in children younger than 3 months unless the situation is judged critical. CDC recommends oseltamivir treatment as soon as possible for hospitalized patients with suspected or confirmed influenza, high-risk outpatients with suspected or confirmed influenza, and those with progressive disease.
- Inhaled zanamivir is FDA-approved for treatment of uncomplicated influenza within 2 days of illness onset in persons 7 years and older and for prevention of influenza in persons 5 years and older. Inhaled zanamivir is not recommended for treatment of influenza in hospitalized patients due to limited data.
- Intravenous peramivir is FDA-approved for the treatment of acute uncomplicated influenza within 2 days of illness onset in persons aged 2 years and older.
Adamantanes (rimantadine and amantadine) are not currently recommended for antiviral treatment or chemoprophylaxis of influenza A because of high levels of resistance among circulating influenza A viruses.
There are no current national shortages of neuraminidase inhibitors (i.e., oseltamivir, zanamivir and peramivir), and manufacturers report they expect to meet projected seasonal demands. If there is difficulty locating oseltamivir for oral suspension, as there has been in some previous seasons, oral suspension can be compounded by a pharmacy from oseltamivir capsules. However, this compounded suspension should not be used for convenience or when oseltamivir oral suspension is commercially available.
More information about compounding an oral suspension from oseltamivir 75 mg capsules can be found at https://www.gene.com/download/pdf/tamiflu_prescribing.pdf
Additional Considerations for Clinicians
- Bacterial Infections: Antibiotics are not effective against influenza virus infection, and early diagnosis of influenza can reduce the inappropriate use of antibiotics if bacterial co-infection is not suspected. However, because certain bacterial infections can produce symptoms similar to influenza and bacterial infections can occur as a complication of influenza, bacterial infections should be considered and appropriately treated, if suspected. In addition, because pneumococcal infections are a serious complication of influenza infection, current pneumococcal vaccine recommendations for adults 65 years of age or older, as well as adults and children at increased risk for invasive pneumococcal disease due to chronic underlying medical conditions, should be followed (see http://www.cdc.gov/vaccines/vpd-vac/pneumo/vac-PCV13-adults.htm and http://www.cdc.gov/vaccines/vpd-vac/pneumo/vacc-in-short.htm for further information).
- Adverse Events and Antiviral Use: The most common adverse events associated with oral oseltamivir include a slightly increased risk of nausea and vomiting as compared to placebo, with nausea occurring in 10% of adults with influenza who received oseltamivir and 6% of people who received placebo in controlled clinical trials (3% and 4%, respectively, in children), and vomiting occurring in 9% of adults with influenza who received oseltamivir and 3% of people who received placebo in controlled clinical trials (15% and 9%, respectively, in children). These symptoms are generally transient and can be mitigated if oseltamivir is taken with food. Adverse events for inhaled zanamivir were not increased as compared to placebo in clinical trials, but cases of bronchospasm have been reported during post marketing; inhaled zanamivir is not recommended for persons with underlying airways disease (e.g., asthma or chronic obstructive pulmonary diseases). For people who received peramivir intravenously or intramuscularly in clinical trials, the most common adverse event was diarrhea, occurring in 8% versus 7% in people who received placebo.
Resources for Patient Education
Results from unpublished CDC qualitative research shows that most people interviewed were not aware that drugs to treat influenza illness are available. A fact sheet for patients is available at http://www.cdc.gov/flu/antivirals/whatyoushould.htm.
Note the following important background information for patients:
- If you get the flu, antiviral drugs are a treatment option.
- It is very important that antiviral drugs are used early to treat hospitalized patients, people with severe flu illness, and people who are at high risk for flu complications because of their age, severity of illness, or underlying medical conditions.
- If you have severe illness or are at high risk of serious flu complications, you may be treated with flu antiviral drugs if you get the flu.
- If you have a high-risk condition, treatment with an antiviral drug can mean the difference between having milder illness instead of very serious illness that could result in a hospital stay.
- Other people also may be treated with antiviral drugs by their doctor this season. Most otherwise-healthy people who get the flu, however, do not need to be treated with antiviral drugs.
- Studies show that flu antiviral drugs work best for treatment when they are started within 2 days of getting sick. However, starting antivirals later can still be helpful for some people.
- If your health care provider thinks you have the flu, your health care provider may prescribe antiviral drugs. A test for flu is not necessary.
- Antibiotics are not effective against the flu. Using antibiotics inappropriately can lead to antibiotic resistance and may expose patients to unwanted side effects of the drug.
- Other practices that may help decrease the spread of influenza include respiratory hygiene, cough etiquette, social distancing (e.g., staying home from work and school when ill, staying away from people who are sick) and hand washing.
Additional Resources
- Summary of Influenza Antiviral Treatment Recommendations for Clinicians: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- Clinical Description and Lab Diagnosis of Influenza: http://www.cdc.gov/flu/professionals/diagnosis/index.htm
- Guidance for Clinicians on the Use of RT-PCR and Other Molecular Assays for Diagnosis of Influenza Virus Infection: http://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm
- Interim Guidance for Influenza Outbreak Management in Long-Term Care Facilities: http://www.cdc.gov/flu/professionals/infectioncontrol/ltc-facility-guidance.htm
- Influenza Virus Testing in Investigational Outbreaks in Institutional or Other Closed Settings: https://www.cdc.gov/flu/professionals/diagnosis/guide-virus-diagnostic-tests.htm
- FDA Influenza (Flu) Antiviral Drugs and Related Information (including package inserts): http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm100228.htm
References
2 Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and Safety of Oseltamivir in Children: Systematic Review and Individual Patient Data Meta-analysis of Randomized Controlled Trials. Clin Infect Dis. 2017 Nov 23. doi: 10.1093/cid/cix1040. [Epub ahead of print]
3 Hsu J, Santesso N, Mustafa R, Brozek J, Chen YL, Hopkins JP, Cheung A, Hovhannisyan G, Ivanova L, Flottorp SA, Saeterdal I, Wong AD, Uyeki TM, Akl EA, Alonso-Coello P, Smaill F, Schūnemann HJ. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012 Apr 3;156(7):512-24.
4 Doll MK, Winters N, Boikos C, Kraicer-Melamed H, Gore G, Quach C. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother. 2017 Nov 1;72(11):2990-3007.
5 Venkatesan S, Myles PR, Leonardi-Bee J, Muthuri SG, Al Masri M, Andrews N, Bantar C, Dubnoy-Raz G, Gérardin P, Koay ESC, Loh TP, Memish Z, Miller E, Oliva ME, Rath BA, Schweiger B, Tanq JW, Tran D, Vidmar T, Waight PA, Nguyen-Van-Tam JS. Impact of Outpatient Neuraminidase Inhibitor Treatment in Patients Infected With Influenza A(H1N1)pdm09 at High Risk of Hospitalization: An Individual Participant Data Metaanalysis. Clin Infect Dis. 2017 May 15;64(10):1328-1334.
6 Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Al Khuwaitir TS, Al Mamun A, Anovadiya AP, Azziz-Baumgartner E, Báez C, Bassetti M, Beovic B, Bertisch B, Bonmarin I, Booy R, Borja-Aburto VH, Burgmann H, Cao B, Carratala J, Denholm JT, Dominguez SR, Duarte PA, Dubnov-Raz G, Echavarria M, Fanella S, Gao Z, Gérardin P, Giannella M, Gubbels S, Herberg J, Iglesias AL, Hoger PH, Hu X, Islam QT, Jiménez MF, Kandeel A, Keijzers G, Khalili H, Knight M, Kudo K, Kusznierz G, Kuzman I, Kwan AM, Amine IL, Langenegger E, Lankarani KB, Leo YS, Linko R, Liu P, Madanat F, Mayo-Montero E, McGeer A, Memish Z, Metan G, Mickiene A, Mikić D, Mohn KG, Moradi A, Nymadawa P, Oliva ME, Ozkan M, Parekh D, Paul M, Polack FP, Rath BA, Rodríguez AH, Sarrouf EB, Seale AC, Sertogullarindan B, Siqueira MM, Skręt-Magierło J, Stephan F, Talarek E, Tang JW, To KK, Torres A, Törün SH, Tran D, Uyeki TM, Van Zwol A, Vaudry W, Vidmar T, Yokota RT, Zarogoulidis P; PRIDE Consortium Investigators, Nguyen-Van-Tam JS.. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014 May;2(5):395-404.
7 Fry AM, Goswami D, Nahar K, Sharmin AT, Rahman M, Gubareva L, Azim T, Bresee J, Luby SP, Brooks WA. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014 Feb;14(2):109-18.
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and controlling diseases and injuries; enhances health decisions by providing credible information on critical health issues; and promotes healthy living through strong partnerships with local, national and international organizations.
Department of Health and Human Services
HAN Message Types
- Health Alert: Conveys the highest level of importance; warrants immediate action or attention. Example: HAN00001(https://emergency.cdc.gov/han/han00001.asp)
- Health Advisory: Provides important information for a specific incident or situation; may not require immediate action. Example: HAN00346(https://emergency.cdc.gov/han/han00346.asp)
- Health Update: Provides updated information regarding an incident or situation; unlikely to require immediate action. Example: HAN00342(https://emergency.cdc.gov/han/han00342.asp)
- Info Service: Provides general information that is not necessarily considered to be of an emergent nature. Example: HAN00345(https://emergency.cdc.gov/han/han00345.asp)
###
This message was distributed to state and local health officers, state and local epidemiologists, state and local laboratory directors, public information officers, HAN coordinators, and clinician organizations.
###
CDC: During week 51 (December 17-23, 2017), influenza activity increased sharply in the United States.
Saturday, December 30th, 2017During week 51 (December 17-23, 2017), influenza activity increased sharply in the United States.
- Viral Surveillance: The most frequently identified influenza virus subtype reported by public health laboratories during week 51 was influenza A(H3). The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Three influenza-associated pediatric deaths were reported.
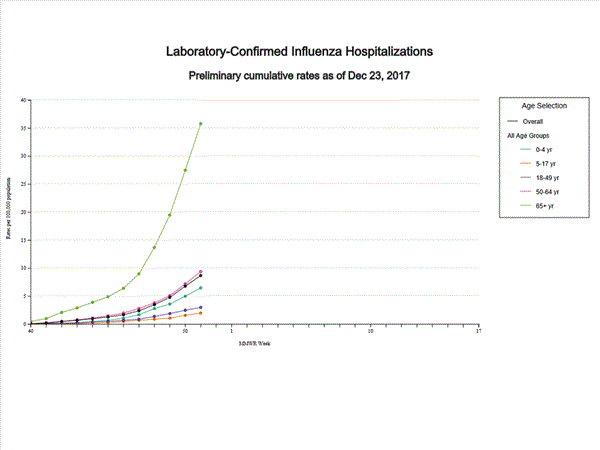
- Influenza-associated Hospitalizations: A cumulative rate of 8.7 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 5.0%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above region-specific baseline levels. Twenty-one states experienced high ILI activity; New York City and five states experienced moderate ILI activity; eight states experienced low ILI activity; 14 states experienced minimal ILI activity; and the District of Columbia, Puerto Rico and two states had insufficient data.
- Geographic Spread of Influenza:The geographic spread of influenza in 36 states was reported as widespread; Puerto Rico and 13 states reported regional activity; one state reported local activity; and the District of Columbia, the U.S. Virgin Islands, and Guam did not report.
During week 49 (December 3-9, 2017), influenza activity increased in the United States.
Saturday, December 16th, 2017During week 49 (December 3-9, 2017), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 49 was influenza A. The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: One influenza-associated pediatric death was reported.
- Influenza-associated Hospitalizations: A cumulative rate of 4.3 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.7%, which is above the national baseline of 2.2%. Seven of the 10 regions reported ILI at or above region-specific baseline levels. Four states experienced high ILI activity; five states experienced moderate ILI activity; New York City, Puerto Rico, and 16 states experienced low ILI activity; 25 states experienced minimal ILI activity; and the District of Columbia had insufficient data.
- Geographic Spread of Influenza:The geographic spread of influenza in 12 states was reported as widespread; Puerto Rico and 26 states reported regional activity; 10 states reported local activity; the District of Columbia, the U.S. Virgin Islands and two states reported sporadic activity; and Guam did not report.


Between 291,000 and 646,000 people worldwide die from seasonal influenza-related respiratory illnesses each year, higher than a previous estimate of 250,000 to 500,000
Friday, December 15th, 2017“….The new estimates use more recent data, taken from a larger and more diverse group of countries than previous estimates. Forty-seven countries contributed to this effort. Researchers calculated annual seasonal influenza-associated respiratory deaths for 33 of those countries (57 percent of the world’s population) that had death records and seasonal influenza surveillance information for a minimum of four years between 1999 and 2015. Statistical modeling with those results was used to generate an estimate of the number of flu-associated respiratory deaths for 185 countries across the world. Data from the other 14 countries were used to validate the estimates of seasonal influenza-associated respiratory death from the statistical models.
Poorest nations, older adults hit hardest by flu
Researchers calculated region-specific estimates and age-specific mortality estimates for people younger than 65 years, people 65-74 years, and people 75 years and older. The greatest flu mortality burden was seen in the world’s poorest regions and among older adults. People age 75 years and older and people living in sub-Saharan African countries experienced the highest rates of flu-associated respiratory deaths. Eastern Mediterranean and Southeast Asian countries had slightly lower but still high rates of flu-associated respiratory deaths.
Despite World Health Organization recommendations to use flu vaccination to help protect people in high-risk populations, few developing countries have seasonal flu vaccination programs or the capacity to produce and distribute seasonal or pandemic vaccines.
Global flu surveillance protects all nations, including U.S.
CDC works with global partners to improve worldwide capacity for influenza prevention and control. CDC has helped more than 60 countries build surveillance and laboratory capacity to rapidly detect and respond to influenza threats, including viruses with the potential to cause global pandemics. These efforts, along with technical support, has helped some partners generate estimates of influenza-associated deaths, which contributed to this global effort.
Global surveillance also provides the foundation for selecting the viruses used to make seasonal flu vaccines each year. This helps improve the effectiveness of flu vaccines used in the United States. Global surveillance also is crucial to pandemic preparedness by identifying viruses overseas that might pose a human health risk to people in the United States.
“This work adds to a growing global understanding of the burden of influenza and populations at highest risk,” says CDC researcher Danielle Iuliano, lead author of The Lancet study. “It builds the evidence base for influenza vaccination programs in other countries.”
The study authors note that these new estimates are limited to flu-associated respiratory deaths and therefore may underestimate the true global impact of seasonal influenza. Influenza infection can create or exacerbate other health factors which are then listed as the cause of death on death certificates, for example cardiovascular disease, diabetes, or related complications. Additional research to estimate non-respiratory causes of flu-associated deaths are ongoing…..”
2017-2018 Influenza Season Week 48 ending December 2, 2017
Saturday, December 9th, 2017During week 48 (November 26-December 2, 2017), overall influenza activity increased slightly in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 48 was influenza A. The percentage of respiratory specimens testing positive for influenza in clinical laboratories declined slightly.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Two influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 3.0 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 2.3%, which is above the national baseline of 2.2%. Regions 1, 4, 6 and 7 reported ILI at or above region-specific baseline levels. Three states experienced high ILI activity; Puerto Rico and three states experienced moderate ILI activity; the District of Columbia and six states experienced low ILI activity; and New York City and 38 states experienced minimal ILI activity.
- Geographic Spread of Influenza:The geographic spread of influenza in seven states was reported as widespread; Puerto Rico and 18 states reported regional activity; 18 states reported local activity; and the District of Columbia, the U.S. Virgin Islands and seven states reported sporadic activity; and Guam did not report.
Risk factors for serious outcomes associated with influenza illness in high- versus low- and middle-income countries.
Thursday, December 7th, 2017“….. In LMIC (low- and middle-income countries), but not in high income countries (HIC), pregnant women, people with HIV/AIDS and children < 5 years old (compared with older children) were at increased risk of a severe outcome. Also, although all patients with neurological conditions were at higher risk of severe outcomes than those without, children were at higher risk than adults and children who lived in a LMIC were at significantly higher risk than those living in HIC. Adults were more likely than children to suffer a severe outcome if they had diabetes or a hematologic condition, were obese or had liver disease. Asthma is a risk factor for hospital admission but not for severe outcomes…..”
Week 47 (November 19-25, 2017): Flu activity increased in the United States.
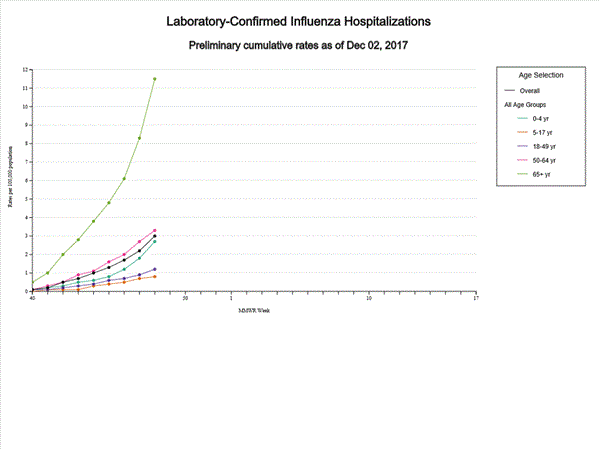
Saturday, December 2nd, 2017During week 47 (November 19-25, 2017), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 47 was influenza A. The percentage of respiratory specimens testing positive for influenza in clinical laboratories is increasing.
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: No influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate of 2.0 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 2.3%, which is above the national baseline of 2.2%. Regions 1, 4, 6 and 7 reported ILI at or above region-specific baseline levels. Three states experienced high ILI activity; one state experienced moderate ILI activity; 10 states experienced low ILI activity; the District of Columbia, New York City and 36 states experienced minimal ILI activity; and Puerto Rico had insufficient data.
- Geographic Spread of Influenza:The geographic spread of influenza in four states was reported as widespread; Guam and 10 states reported regional activity; Puerto Rico and 24 states reported local activity; and the District of Columbia, the U.S. Virgin Islands and 12 states reported sporadic activity.
<!–
–>
Reports from Australia have caused mounting concern about flu season in the Northern Hemisphere, with record-high numbers of laboratory-confirmed flu notifications and outbreaks and higher-than-average numbers of hospitalizations and deaths
Thursday, November 30th, 2017Chasing Seasonal Influenza — The Need for a Universal Influenza Vaccine
November 29, 2017
DOI: 10.1056/NEJMp1714916
“…..Seasonal influenza epidemics cause 3 million to 5 million severe cases and 300,000 to 500,000 deaths globally each year…… The United States alone sees 140,000 to 710,000 influenza-related hospitalizations and 12,000 to 56,000 deaths each year, with the highest burden of disease affecting the very young, the very old, and people with coexisting medical conditions….”
“…..Another factor that may alter the effectiveness of influenza vaccines is the substrate used to produce them. In the United States, most influenza-vaccine viruses are propagated in eggs, although a small proportion are produced either in cell culture or by expressing specific viral proteins using recombinant DNA technologies. During the egg-based production process, the vaccine virus acquires amino acid changes that facilitate replication in eggs, notably changes in the hemagglutinin (HA) protein that mediates receptor binding.3 Since the influenza HA is the primary target of neutralizing antibodies, small modifications in this protein can cause antigenic changes in the virus and decrease vaccine effectiveness. Egg adaptation has been postulated to contribute to low vaccine effectiveness, particularly with influenza A (H3N2) viruses; however, the true impact is largely unknown…….”
“….the CDC estimates that influenza vaccination averted 40,000 deaths in the United States between the 2005–2006 and 2013–2014 seasons….”
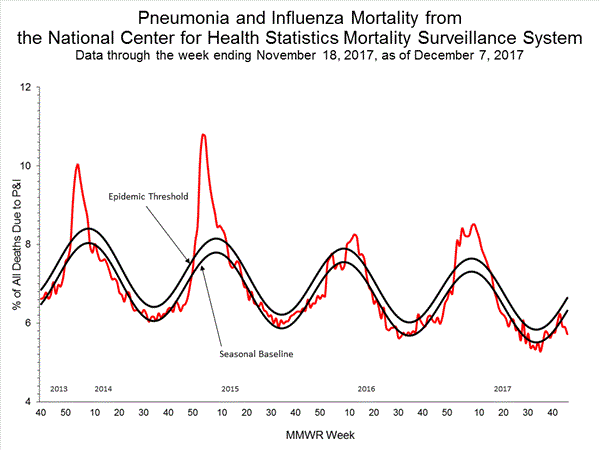
2017-2018 Influenza Season Week 46 ending November 18, 2017
Wednesday, November 29th, 2017Synopsis:
During week 46 (November 12-18, 2017), influenza activity increased in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 46 was influenza A. The percentage of respiratory specimens testing positive for influenza in clinical laboratories is increasing.
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
- Influenza-associated Pediatric Deaths: Five influenza-associated pediatric deaths were reported, one of which occurred during the 2016-17 season.
- Influenza-associated Hospitalizations: A cumulative rate of 1.4 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) was 2.0%, which is below the national baseline of 2.2%. Regions 1, 2, 4 and 6 reported ILI at or above region-specific baseline levels. Two states experienced high ILI activity, one state experienced moderate ILI activity, New York City and 4 states experienced low ILI activity, the District of Columbia and 43 states experienced minimal ILI activity, and Puerto Rico had insufficient data.
- Geographic Spread of Influenza:The geographic spread of influenza in two states was reported as widespread; Guam and six states reported regional activity; 20 states reported local activity; the District of Columbia, the U.S. Virgin Islands and 21 states reported sporadic activity; one state reported no activity; and Puerto Rico did not report.