Archive for the ‘Strategic National Stockpile’ Category
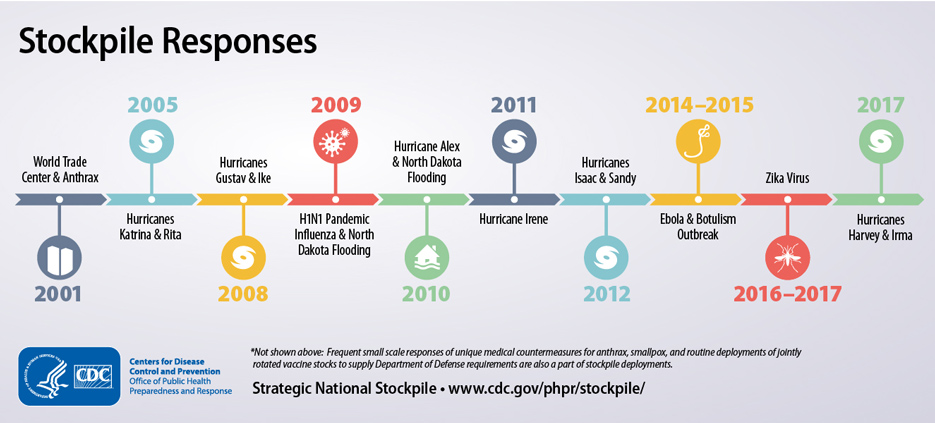
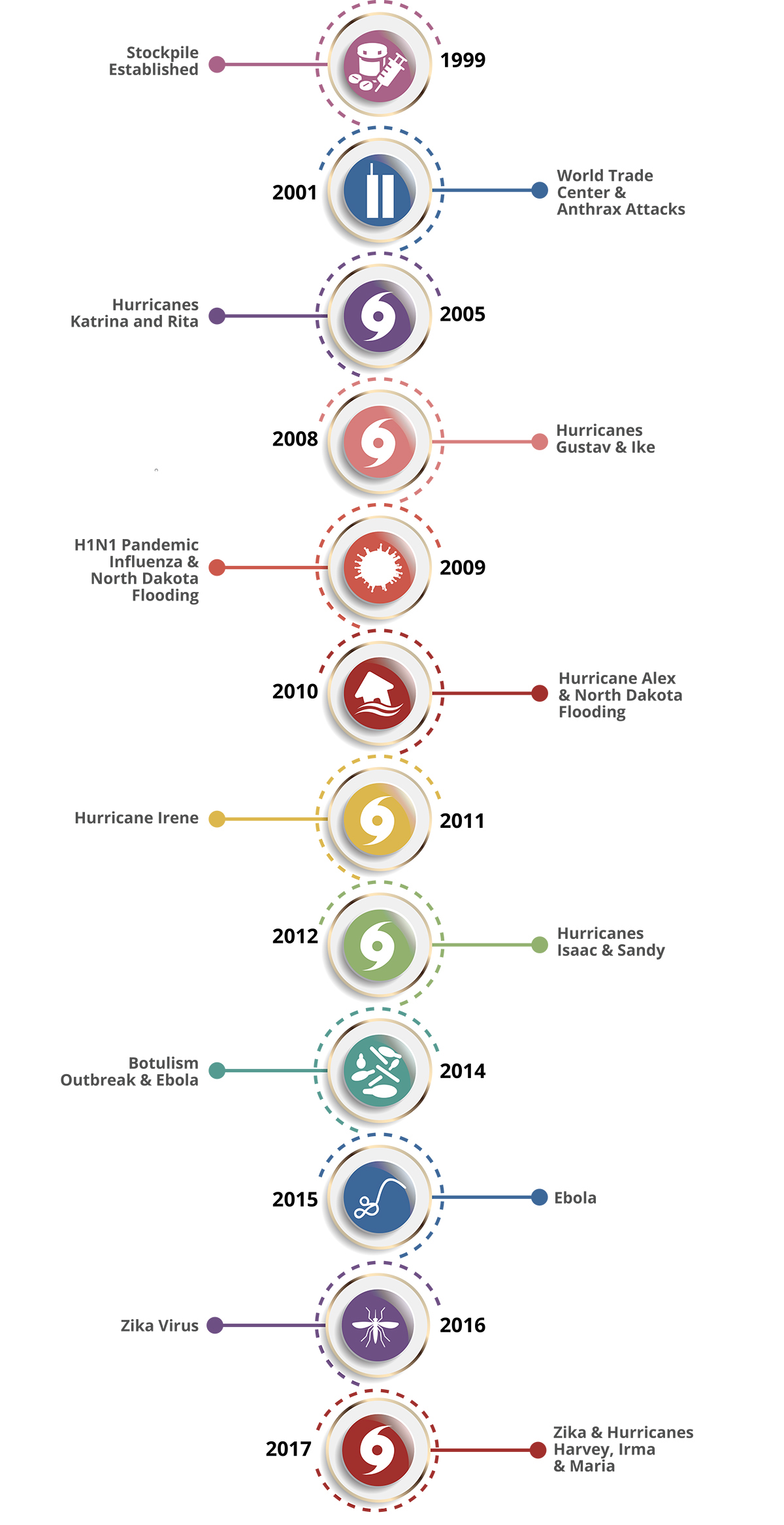
The Early Years of the Strategic National Stockpile (SNS)
Monday, October 21st, 2019“……..In just 20 years, the SNS – now managed by the U.S. Department of Health and Human Services’ (HHS) Assistant Secretary for Preparedness and Response (ASPR) – has grown to a $7 billion enterprise poised to respond to a variety of public health threats. These threats include anthrax, botulism, smallpox, plague, tularemia and viral hemorrhagic fevers, as well as emerging infectious diseases, pandemic influenza, natural disasters, and other chemical, biological, radiological, and nuclear incidents……..”
History of the Strategic National Stockpile
Thursday, September 12th, 2019The National Pharmaceutical Stockpile was created in 1999 to ensure the nation’s readiness against potential agents of bioterrorism like botulism, anthrax, smallpox, plague, viral hemorrhagic fevers, and tularemia. The mission was to assemble large quantities of essential medical supplies that could be delivered to states and communities during an emergency within 12 hours of the federal decision to use the stockpile.
The September 11, 2001, terrorist attacks prompted federal legislation and directives to strengthen public health emergency readiness. In 2003, the National Pharmaceutical Stockpile was renamed Strategic National Stockpile.
Today, the Strategic National Stockpile works with governmental and nongovernmental partners to upgrade the ability to respond to a national public health emergency, ensuring that federal, state, and local agencies are ready to receive, and stage and distribute products.
Since its beginning, the stockpile has responded to multiple large-scale emergencies including floods, hurricanes, and influenza pandemics. It has also supported various small-scale deployments for the treatment of individuals with life-threatening infectious diseases like anthrax, smallpox, and botulism.
Stockpile Products in the Strategic National Stockpile
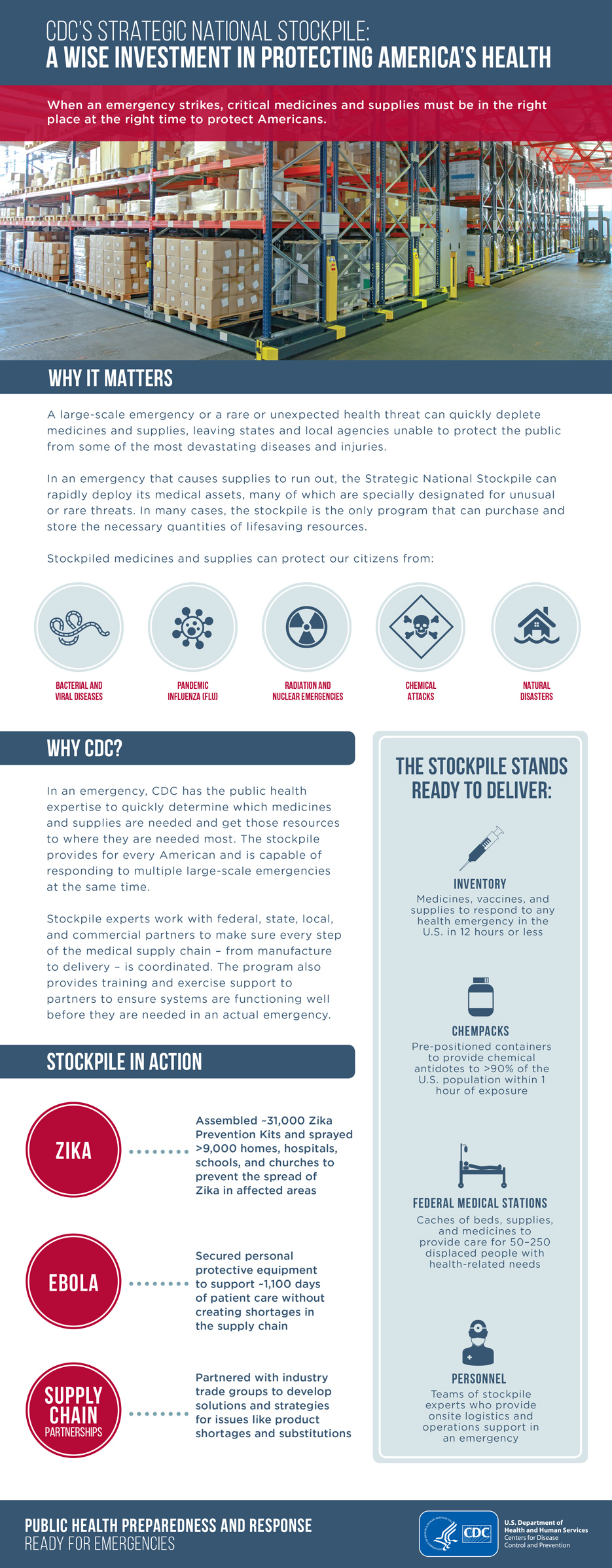
Thursday, September 12th, 2019The majority of stockpile assets are held in storage and kept as managed inventory. Maintaining a supply of medications and medical supplies for specific health threats allows the stockpile to respond with the right product when a specific disease or agent is known.
Products in the stockpile may require an Emergency Use Authorization, which is granted by the U.S. Food and Drug Administration (FDA). The authorization allows for the emergency use of an unapproved medical product (e.g., drugs, vaccines, and devices) or unapproved use of an approved medical product to diagnose, treat, or prevent serious or life-threatening diseases or conditions for which no adequate, FDA-approved alternative is available.
If a community experiences a large-scale public health incident in which the disease or agent is unknown, the first line of support from the stockpile is to send a broad-range of pharmaceuticals and medical supplies. Contents are pre-packed and configured in transport-ready containers for rapid delivery anywhere in the United States within 12 hours of the federal decision to deploy. Each package contains 50 tons of emergency medical resources.
The stockpile has medicines and supplies stored in strategically located warehouses throughout the country ready for deployment. Immediately shipping a variety of items to the affected state allows authorities to begin or sustain response efforts. All states have plans to receive and distribute these medical countermeasures quickly to local jurisdictions.

CHEMPACK
CHEMPACKs are containers of nerve agent antidotes placed in secure locations in local jurisdictions around the country to allow rapid response to a chemical incident. These medications treat the symptoms of nerve agent exposure and can be used even when the actual agent is unknown.
Because these antidotes must be administered quickly, the CHEMPACK team maintains 1,960 containers strategically placed in more than 1,340 locations in the United States. More than 90 percent of the U.S. population is within 1 hour of a CHEMPACK location. Most are located in hospitals or fire stations selected by local authorities to support a rapid hazmat response and can be accessed quickly if hospitals or first responders need them.
Nerve Agent EUA Information
On April 11, 2017, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to permit the emergency use of the 2 mg atropine auto-injector, manufactured by Rafa Laboratories, Ltd. On May 23, 2017, FDA amended the EUA to also permit the emergency use of pediatric strengths (i.e., 0.5 mg and 1 mg) of this atropine auto-injector. On January 24, 2018, FDA granted another EUA amendment to permit auto-injector administration through clothing. These products are included in CHEMPACK containers located across the United States. This specific atropine auto-injector is authorized for the initial treatment of symptoms of known or suspected poisoning in individuals exposed to nerve agents or certain insecticides (organophosphorus and/or carbamate). The original letter of authorization describes the scope and conditions of EUA that apply to all three strengths (0.5 mg, 1 mg and 2 mg) of this atropine auto-injector; the amendment letters are addenda to the original letter of authorization. The amended fact sheets dated January 24, 2018 (version 3) for healthcare providers and patients and caregivers are the current versions and available through FDA’s EUA website.
Federal Medical Stations
Somewhere between a temporary shelter and temporary hospital, a Federal Medical Station is a non-emergency medical center set up during a natural disaster to care for displaced persons with special health needs—including those with chronic health conditions, limited mobility, or common mental health issues—that cannot be met in a shelter for the general population during an incident.
The modular and rapidly deployable reserve of beds, supplies, and medicines provides equipment to care for 50–250 people for 3 days before resupply is needed. Flexible and scalable, it can be tailored to meet the requirements of each incident and has the ability to increase local healthcare capabilities in mass casualty events or in response to potential public health threats.
Federal Medical Stations operate through the cooperation of federal, state, and local authorities and require pre-planning and pre-identification of potential locations, based on specific selection criteria. Prior to an emergency event, state, local, tribal, and territorial agencies collaborate with the U.S. Department of Health and Human Services Regional Emergency Coordinators to survey sites and identify any gaps that may need addressing during a response.
Emergent BioSolutions today announced that it was awarded a contract worth about $2 billion over the next 10 years to deliver the ACAM200 smallpox vaccine to the Strategic National Stockpile.
Wednesday, September 4th, 2019
“…….About ACAM2000 Vaccine
ACAM2000 vaccine is the primary smallpox vaccine designated for use in a bioterrorism emergency, with almost 269 million doses having been supplied to the U.S. Strategic National Stockpile. ACAM2000 vaccine is also licensed in Australia and Singapore and is currently stockpiled both in the U.S. and internationally.
ACAM2000 is indicated for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection.
The labeling for ACAM2000 contains a contraindication for individuals with severe immunodeficiency. Severe localized or systemic infection with vaccinia (progressive vaccinia) may occur in persons with weakened immune systems. Individuals with severe immunodeficiency who are not expected to benefit from the vaccine should not receive ACAM2000. The risk for experiencing severe vaccination complications must be weighed against the risk for experiencing a potentially fatal smallpox infection.
Additionally, there are warnings and precautions for myocarditis, pericarditis, encephalitis, encephalomyelitis, encephalopathy, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson Syndrome), eczema vaccinatum resulting in permanent sequelae or death, ocular complications; blindness and fetal death have occurred following either primary vaccination or revaccination with live vaccinia virus smallpox vaccines. These risks are increased in certain individuals and may result in severe disability, permanent neurological sequalae and/or death.
Please see full Prescribing Information for full boxed warning and additional safety information…..”
Project BioShield over 15 years of progress
Saturday, August 17th, 2019ASPR
Project BioShield Evolution: Fifteen Years of Bridging the ‘Valley of Death’ in the Medical Countermeasures Pipeline
Author: By Dr. Robert Kadlec, Assistant Secretary for Preparedness and Response
Published Date: 7/17/2019 12:15:00 PM
Category: Innovations; Medical Countermeasures; National Health Security;
Saving lives in a public health emergency requires cutting-edge medical countermeasures: medications, vaccines, diagnostics, and more. In some types of emergencies, like an act of bioterrorism, some of those medical products have no commercial market. People don’t use an anthrax antitoxin every day – thank goodness. Yet that lack of commercial market also means pharmaceutical or biotech companies had only one possible buyer for their products: the federal government for stockpiling.
In 2004, Congress passed the Project BioShield Act to create a market for products necessary for disaster response but with limited or no commercial market. The Act provides HHS with a multi-year special reserve fund to support late-stage development and manufacturing, and the financial resources to buy these life-saving medical products for the American people to use in public health emergencies. In this way, Project BioShield is a critical part of the U.S. strategy for biodefense and our commitment to the American people.
Last month, Congress took action to further strengthen Project BioShield as part of the Pandemic and All-Hazards Preparedness and Advancing Innovation Act of 2019 (PAHPAIA). PAHPAIA increases the budget authorization and provides ten-year funding for product development. We know next-generation medical countermeasures aren’t developed overnight – in fact, getting a product across the finish line takes many years. Multi-year funding helps BARDA continue building the strong public-private partnerships needed to spur innovation and provide the private sector with the stability needed to produce potentially lifesaving medical countermeasures.
For example, smallpox is one of the most consequential infectious diseases in human history, responsible for nearly 300 million deaths in the 20th century alone. It is also a high-priority threat requiring federal agencies to develop strategies and countermeasures against this threat. Thanks to our partners at NIAID and DoD and the support of Project BioShield, last year our industry partner was issued an FDA approval for a treatment for smallpox via the animal rule. At present, there are now over 2 million treatment courses in ASPR’s Strategic National Stockpile to protect Americans in the event of a smallpox national security emergency.
However, we have found over the past 15 years the most practical and cost-effective approach is, whenever possible, to look at products that not only can be used for emergencies, but also have uses in daily medical care, such as burn care, the radiation effects cancer patients encounter, or seizures.
In some cases, we’ve worked with companies to expand indications for existing products. For example, three medical countermeasures are now FDA-approved to treat patients suffering bone marrow and blood cell damage from acute radiation syndrome. All three of these products were already licensed drugs used to treat patients undergoing radiation therapy for cancer. Project BioShield funding was used to conduct the critical studies needed to expand the indications for these products so they could also be used to treat the damage caused by acute radiation syndrome in a radiation emergency. Using such drugs is helpful in emergencies because healthcare facilities already stock the drugs, and clinicians are already familiar with using them.
We are using Project BioShield to support other new products with commercial market potential. For example, we supported a large study of a seizure treatment because seizures are one of the potentially deadly effects of nerve agents. The product is used commonly in preparing patients for surgery and for epileptic seizures; thanks to Project BioShield, the product is now approved as an antiseizure medication for status epilepticus and has been added to the Strategic National Stockpile for use in a chemical emergency response.
For 15 years, BARDA has been proud to partner with industry to develop cutting-edge medical countermeasures. Our country is better prepared to respond to health security threats because of Project BioShield. We look forward continued collaboration as we work to develop and produce medical countermeasures that can be used to save lives in the event of an emergency.
Strategic National Stockpile Graphics: Emergency Medical Supplies Protecting The Public’s Health
Sunday, September 10th, 2017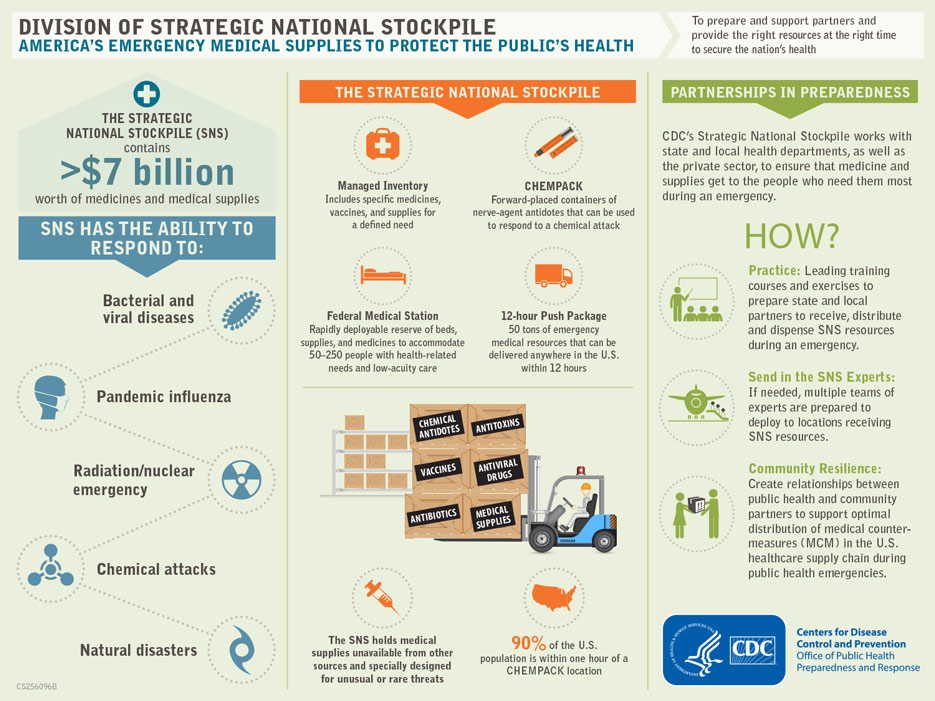
Strategic National Stockpile & its role during Harvey
Sunday, September 10th, 2017The Strategic National Stockpile supported the federal government’s response to Hurricane Harvey in Texas and Louisiana with Federal Medical Stations (FMS), personnel strike teams, and liaison officers. Overall, the stockpile deployed 6 FMS sets with 250 beds each, 12 FMS strike team members, 1 liaison to the Incident Response Coordination Team in Texas and 3 liaisons to the Department of Health and Human Services (HHS) Secretary’s Operations Center in Washington, D.C. Two FMS were set up and operational in Houston – one at the George R. Brown Convention Center and the other at the NRG Center. An additional four FMS were ready on standby – two in Texas and two in Louisiana – so they could be set up at a moment’s notice. Approximately 45 staffers also served in the stockpile’s team room at CDC headquarters.

Federal Medical Station setup at George R. Brown Convention Center in Houston, Texas.
Somewhere between a temporary shelter and temporary hospital, a Federal Medical Station is a non-emergency medical center set up during a natural disaster to care for displaced persons with special health needs—including those with chronic health conditions, limited mobility, or common mental health issues—that cannot be met in a shelter for the general population during an incident.
The modular and rapidly deployable reserve of beds, supplies, and medicines provides equipment to care for 50–250 people for three days before resupply is needed. Flexible and scalable, it can be tailored to meet the requirements of each incident and has the ability to increase local healthcare capabilities in mass casualty events or in response to potential public health threats.
Federal Medical Stations operate through the cooperation of federal, state, and local authorities and require pre-planning and pre-identification of potential locations, based on specific selection criteria. Prior to an emergency event, state, local, tribal, and territorial agencies collaborate with the Department of Health and Human Services regional emergency coordinators to survey sites and identify any gaps that may need addressing during a response.
The stockpile also deploys the Federal Medical Station Strike Team, which is a group of technical specialists with specific, in-depth knowledge of the stockpile and supply operations. This specialized team can assist federal and state responders, clinicians, and public health staff with identifying a suitable facility, receiving and staging equipment, and on-the-spot training to volunteers who will assemble the equipment (e.g., beds and nurses stations). The strike team can also request additional material as needed.