Tunisia: Envelopes delivered to politicians, journalists and syndicate members contained the Anthrax toxin.
March 5th, 2019“……Twenty public figures were targeted in this horrifying terrorist plot, including 10 prominent politicians, seven journalists and activists in syndicates and human rights, they continued.
Moreover, they said that the terrorist groups have shifted their tactics after security measures against them have limited their activity.
For years, they have relied on armed attacks, but they are now forced to resort to poisoning their victims, said the security agencies…..”
Types of Anthrax(https://www.cdc.gov/anthrax/basics/types/index.html)
Yesterday’s Tornadoes – AL and GA
March 5th, 2019Current Situation: Multiple tornadoes touched down Sunday afternoon/evening in AL and GA. NWS confirmed an EF-4 tornado in Lee County, AL. Hundreds of homes and multiple public buildings have been damaged and dozens of transportation routes have been impacted in eastern AL and western GA.
Impacts:
- Food, Water and Shelter
- • Shelters: (ARC Midnight shelter count, 6:00 a.m. EST) ₒ AL: 2 open w/ 12 occupants ₒ GA: 2 open w/ 17 occupants Health and Medical
- • Fatalities/Injuries: ₒ AL: 23 confirmed fatalities; approx. 30 people missing (Region IV SPOTREP) ₒ GA: No reported fatalities
- Energy: Minimal power outages across the impacted area (EAGLE-I, as of 6:45 a.m. EST)*
- Transportation
- • AL: Multiple aircraft and hangars damaged at airport in Barbour County; several roads reported impassable in Lee County
- State and Local Response:
- • AL EOC at Partial Activation; Governor extended a State of Emergency
- • Governor requested Expedited Major Disaster Declaration
- • GA EOC at Partial Activation; Governor declaring a State of Emergency
- • Damage assessments with state/local officials ongoing
- FEMA Region IV Response:
- • RWC at Steady State, continues to monitor
- • IMAT-1 deployed to AL EOC
- • IMAT-2 demobilized from GA and is Partially Mission Capable
- • LNO deployed to AL; LNO demobilized from GA
FDA’s Critical Role in Ensuring Supply of Influenza Vaccine
March 5th, 2019The flu vaccine you get at your doctor’s office or pharmacy is the year-round work of highly skilled microbiologists, epidemiologists, physicians and other public health experts.
Sound complicated? It is.
The U.S. Food and Drug Administration (FDA) and the U.S. Department of Health and Human Services (HHS) are working toward developing new and better technologies for producing flu vaccines. As new strains of flu viruses emerge, the FDA works in close coordination with sister agencies, such as the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH), encouraging manufacturers to develop vaccines that will successfully protect us from influenza disease, which can be a very serious illness.
The more diverse the influenza vaccine supply, the better we can respond to flu public health emergencies in a timely manner. All U.S. licensed flu vaccines have been evaluated and determined to be safe and effective by the FDA.
There is often more than one type of influenza virus circulating each season, so influenza vaccines are formulated to target three or four of the most likely influenza viruses of the season: two influenza A types (H1N1 and H3N2) and one (trivalent vaccine formulation) or two (quadrivalent vaccine formulation) types of influenza B.
Influenza Vaccines and How They Are Made
Influenza (flu) vaccine works by triggering your immune system to produce antibodies that help the body prevent the flu.
Most of the U.S. influenza vaccine supply is made using an egg-based production process. In this method of making influenza vaccines, manufacturers use fertilized eggs to grow flu viruses. After about six months of laboratory work and manufacturing, those viruses are incorporated into that season’s flu vaccine. While this method produces safe and effective vaccines, FDA, HHS, and manufacturers have been working on different technologies to increase the influenza vaccine supply, making sure to still maintain safety and effectiveness. Some technologies have the potential to produce these vaccines faster and less labor-intensively.
For example, more recent approvals include influenza vaccines that do not use an egg-based production process for manufacturing. In addition, quadrivalent vaccines, which protect against two influenza A strains and two influenza B strains, have been available for several years. Quadrivalent vaccines provide protection against the two lineages of influenza B strains, both of which circulate each flu season. This is important, particularly for preschool and school-age children who get Influenza B more often than adults do. Influenza B also causes more complications and fatalities in children than adults.
In addition, the FDA has approved a high-dose and an adjuvanted vaccine specifically for people ages 65 and older, who typically bear the greatest burden of severe influenza disease and account for most of influenza-related hospitalizations and deaths. Adjuvants are incorporated into some vaccine formulations to enhance the immune response of the vaccinated individual.
Making the Flu Vaccine: A Year-Round Effort
The job of producing a new vaccine for the next flu season starts well before the current flu season ends. For the FDA, it’s a year-round initiative.
The composition of vaccines for the prevention of other infectious diseases stays the same year after year. In contrast, flu viruses are constantly evolving. And the flu viruses that circulate causing disease in people, often change from one year to another. So, every year, there is a need for a new flu vaccine. To that end, FDA, World Health Organization (WHO), CDC, and other partners collaborate by collecting and reviewing data on the circulating strains of influenza from around the world to identify those likely to cause the most illness in the upcoming flu season.
In late February/early March — well before the new flu season begins — an FDA advisory committee reviews data about which flu viruses have caused disease in the past year, how the viruses are changing, and disease trends so they can recommend the three or four flu strains to include in the trivalent and quadrivalent influenza vaccines for the U.S in the upcoming flu season.
Once the strains are selected, vaccine manufacturers begin the manufacturing process to include the newly selected flu strains in their FDA-approved vaccines. The different flu virus strains are combined to formulate the vaccine into standard dosages. The vaccine is then filled into vials, syringes and, for the nasal vaccine, sprayers. Both egg-based and non-egg-based manufacturing methods for FDA-approved flu vaccines require high-tech processes and manufacturing facilities that have been inspected by the FDA. Vaccine manufacturers must submit applications to the FDA to include the new flu strains in their FDA-approved vaccines.
In its own laboratories, the FDA also produces materials that are critical for making the vaccine. These include generating candidate vaccine virus strains suitable for further vaccine manufacture and producing the critical potency reagents, which are materials needed to test the vaccines for potency and identity (to ensure standardization) before the FDA approves the new formulation of the approved seasonal influenza vaccines for U.S. distribution.
The FDA is also responsible for ensuring that released lots of influenza vaccines meet appropriate standards. Each vaccine undergoes quality control tests, including testing for sterility. Manufacturers submit the results of their testing, along with sample vials from each lot to the FDA for “lot release.” The FDA typically begins releasing lots of flu vaccines in late summer. Lot release can continue into early fall. Once lots are released, manufacturers distribute the vaccine throughout the United States for use by the public.
Flu seasons and severity are unpredictable. Annual vaccination is the best way to prevent the flu for people ages 6 months and older.
An annual immunization with flu vaccine is the most effective and safest way for most of us to reduce our risk of getting the flu and spreading it to others. When more people get vaccinated, it is less likely that the flu viruses will spread through a community, making us all healthier.
Updated: March 1, 2019
MERS-CoV in Oman: 8 new cases
March 5th, 2019Between 12 and 18 February 2019, the National IHR Focal Point of Oman reported eight additional cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Four cases were reported from South Sharquia governorate, and four cases were reported from North Batinah governorate where a MERS-CoV cluster was recently identified. Details of the additional eight cases can be found in the attached excel sheet.
Since 27 January 2019, a total of 13 MERS cases were reported from Oman, including nine from North Batinah (five cases were previously reported in the Disease outbreak News 11 February 2019) and four from South Sharquia.
An investigation into the exposure history to known risk factors in the 14 days prior to symptom onset in all eight cases is currently ongoing.
From 2012 through 18 February 2019, a total of 2357 laboratory-confirmed cases of MERS, along with 820 associated deaths, have been reported to WHO globally. The first reported MERS infection in Oman was reported in 2013. Since then, a total of 24 cases and seven deaths have been reported.
The global number reflects the total number of laboratory-confirmed cases reported to WHO under IHR to date. The total number of deaths includes the deaths that WHO is aware of to date through follow-up with affected member states.
Public Health response
Whole genome sequencing of available human specimens of patients is being conducted. As of 21 February, all identified contacts (family and health workers) of confirmed MERS patients have been screened, including 69 contacts from North Batinah patients and 57 contacts from South Sharqiyah patients. Tracing and follow-up of these contacts is ongoing by the Ministry of Health for 14 days from the last date of exposure as per WHO and national guidelines for MERS-CoV. All contacts have been sampled and have tested negative for MERS-CoV by RT-PCR.
The Ministry of Agriculture has conducted an investigation of dromedaries at the farms of one of the patients. Results of this investigation are pending.
The Ministry of Health has strengthened infection prevention and control measures in emergency departments, especially in triaging areas. Health education and awareness materials were produced and disseminated to health care staff, patients and visitors.
Family members of MERS-CoV infected patients have been contacted and advised about the virus and on measures to ensure personal protection to prevent infection. Efforts in improving public health awareness among the general public has been strengthened through greater messaging in the media.
WHO risk assessment
Infection with MERS-CoV can cause severe disease resulting in high morbidity and mortality. Humans are infected with MERS-CoV from direct or indirect contact with infected dromedary camels. MERS-CoV has demonstrated the ability to transmit between humans, especially from close unprotected contact with infected patients. So far, the observed non-sustained human-to-human transmission has occurred mainly in health care settings.
The notification of these additional cases does not change WHO’s overall risk assessment of MERS-CoV. WHO expects that additional cases of MERS infection will be reported from the Middle East, and that cases will continue to be exported to other countries by individuals who might acquire the infection after exposure to dromedary camels, animal products (e.g. consumption of camel’s raw milk), or humans (e.g. in a health care setting). WHO continues to monitor the epidemiological situation and conducts risk assessment based on the latest available information.
WHO advice
Based on the current situation and available information, WHO encourages all Member States to continue their surveillance for acute respiratory infections and to carefully review any unusual patterns.
Infection prevention and control measures are critical to prevent the possible spread of MERS-CoV between people in health care facilities. It is not always possible to identify patients with MERS-CoV infection early because like other respiratory infections, the early symptoms of MERS are non-specific. Therefore, healthcare workers should always apply standard precautions consistently with all patients, regardless of their diagnosis. Droplet precautions should be added to the standard precautions when providing care to patients with symptoms of acute respiratory infection; contact precautions and eye protection should be added when caring for probable or confirmed cases of MERS infection; airborne precautions should be applied when performing aerosol generating procedures.
Early identification, case management and isolation, together with appropriate infection prevention and control measures can prevent human-to-human transmission of MERS-CoV.
WHO recommends that comprehensive identification, follow up and testing of all contacts of MERS patients be conducted, if feasible, regardless of the development of symptoms since approximately 20% of all reported MERS infections have been reported as mild or asymptomatic. The role of asymptomatic MERS infection in transmission is not well understood. However, reports of transmission from an asymptomatic MERS infected patient to another individual have been documented.
MERS causes more severe disease in people with underlying chronic medical conditions such as diabetes, renal failure, chronic lung disease, and immunocompromised persons. Therefore, these people should avoid close contact with dromedary camels when visiting farms, markets, or barn areas where the virus is known to be potentially circulating. General hygiene measures, such as regular hand washing before and after touching animals and avoiding contact with sick animals, should be adhered to.
Food hygiene practices should be observed. People should avoid drinking raw camel milk or camel urine, and refrain from eating meat that has not been properly cooked.
WHO does not advise special screening at points of entry with regards to this event, and does not currently recommend the application of any travel or trade restrictions at this time.
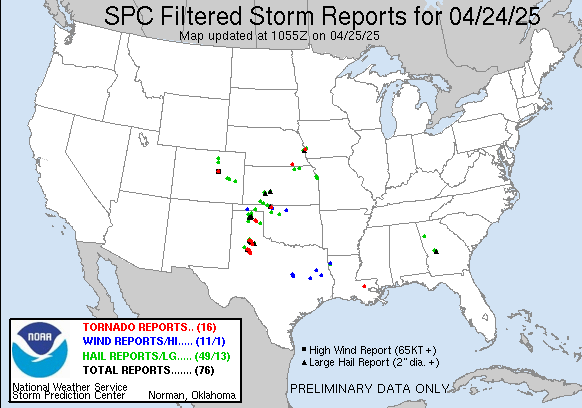
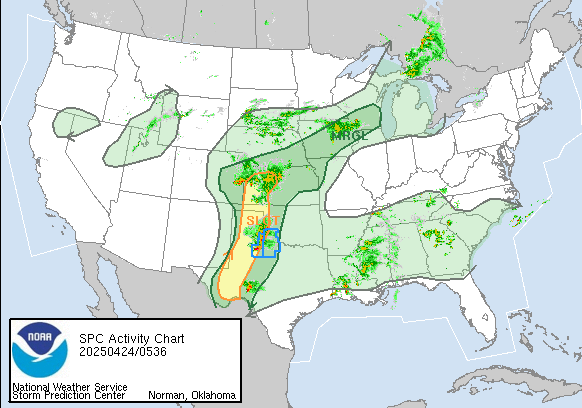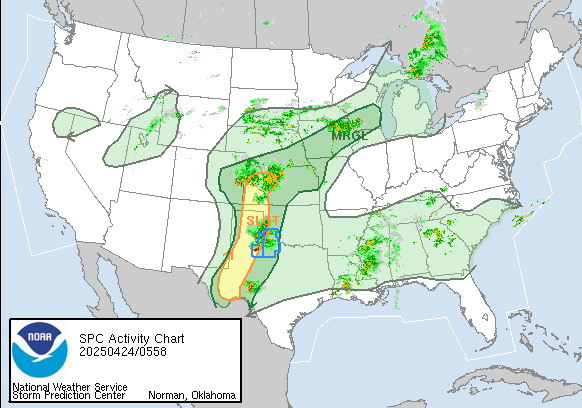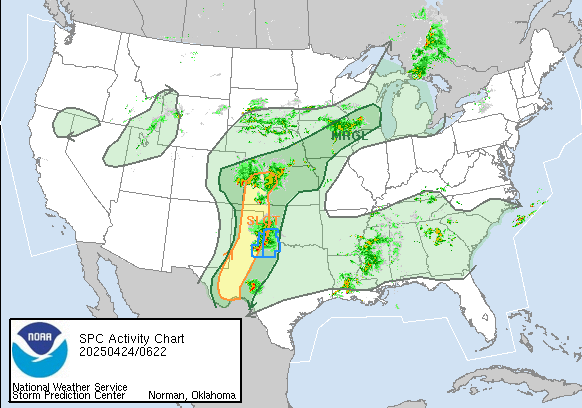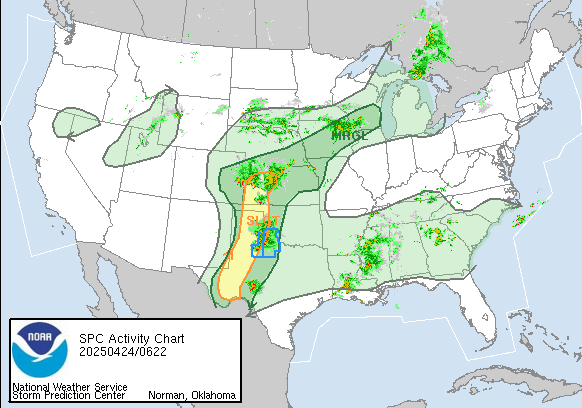
Tornado Reports for 3/3/2019
March 4th, 2019
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Storm Reports page is organized based on reports received from 1200 UTC to 1159 UTC the next day. For example, storm report page for 20150430 covers reports from 20150430 at 1200 UTC to 20150501 at 1159 UTC. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Full report in comma-separated values (CSV) format and in KML format. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Full filtered report in comma-separated values (CSV) format and in KML format. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KML files are created with time-enabled placemarks compatible with Google Earth Time Slider. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Raw full report in comma-separated values (CSV) format. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fields marked UNK are unknown. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All Times UTC. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wind Gusts in MPH. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hail Sizes in 1/100 of an Inch (175 = 1.75″) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LAT/LON in decimal degrees to two decimals, see SPC FAQ for more info. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| List of Weather Forecast Office 3-letter IDs appear in the report comments section. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Major Storm Moves Across the Country Followed by Bitter Cold
March 3rd, 2019
Short Range Forecast Discussion
NWS Weather Prediction Center College Park MD
259 AM EST Sun Mar 03 2019
Valid 12Z Sun Mar 03 2019 – 12Z Tue Mar 05 2019
…Heavy snow possible from the Central Appalachians to Northern New
England…
…Heavy rain possible over the Southeast…
…Slight risk of severe thunderstorms over the Southeast into Central
Gulf Coast…
…Temperatures will be 20 to 45 degrees below normal over the High Plains
into the Mississippi Valley…
A developing storm over the Southern Mid-Atlantic will move northeastward
to just off Southern New England by Monday and moving into the Canadian
Maritimes by Monday evening. The system will produce snow over parts of
the Central Appalachians that will expand into the Northern Mid-Atlantic
by Sunday afternoon. The snow will expand into the Northeast by Sunday
evening and continue over the region through Monday evening. In addition,
showers and thunderstorms will develop over parts of the Southern Plains
into the Lower Mississippi and Tennessee Valleys. Showers and
thunderstorms will also develop over parts of the Southern Mid-Atlantic.
The showers and thunderstorms will move eastward off the Southeast Coast
by Monday morning.
Meanwhile, upper-level energy over the Middle Mississippi Valley into
parts of the Southern High Plains will produce snow over parts of the
Central Rockies eastward into the Middle Mississippi Valley that will move
eastward into the Ohio Valley ending overnight Sunday. Disorganized
upper-level energy will move eastward from the Pacific into the Texas
Panhandle by Tuesday. The energy will aid in producing rain and higher
elevation snow over parts of California eastward to the Central Rockies
through Monday evening.
Ziegenfelder

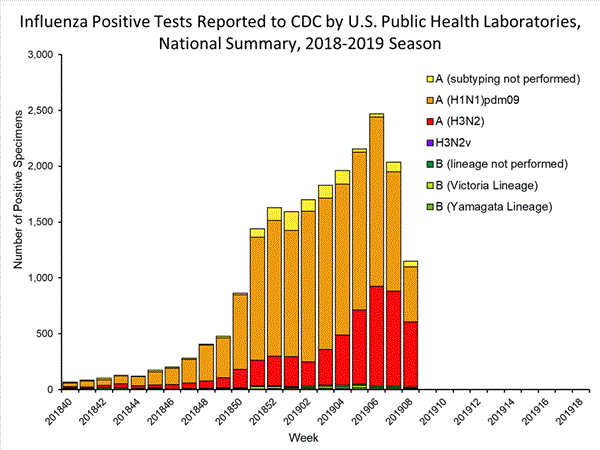
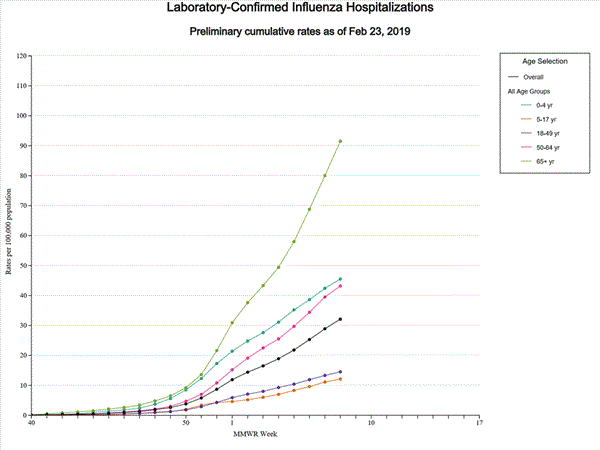
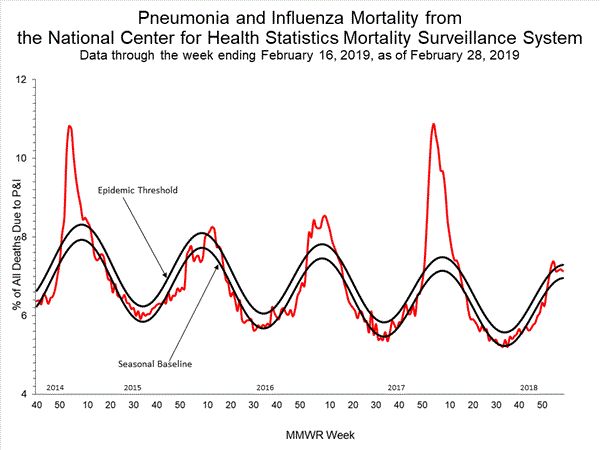
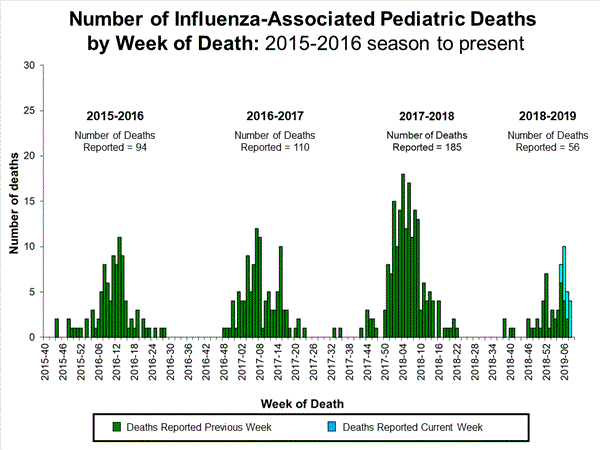
2018-2019 Influenza Season Week 8 ending February 23, 2019: Influenza activity remains elevated in the United States.
March 3rd, 2019UNICEF: Global cases of measles are surging to alarmingly high levels, led by 10 countries accounting for more than 74% of the total increase.
March 3rd, 2019Countries with ten highest increases in cases between 2017 and 2018
| Ukraine | 30,338 |
| Philippines | 13,192 |
| Brazil | 10,262 |
| Yemen | 6,641 |
| Venezuela | 4,916 |
| Serbia | 4,355 |
| Madagascar | 4,307 |
| Sudan | 3,496 |
| Thailand | 2,758 |
| France | 2,269 |