Archive for May, 2016
WHO Emergency Committee: The urban yellow fever (YF) outbreaks in Angola and the Democratic Republic of the Congo is a serious public health event but does not at this time constitute a Public Health Emergency of International Concern (PHEIC).
Friday, May 20th, 2016Meeting of the Emergency Committee under the International Health Regulations (2005) concerning Yellow Fever
An Emergency Committee (EC) regarding yellow fever was convened by the Director-General under the International Health Regulations (2005) (IHR 2005) by teleconference on 19 May 2016, from 13:00 to 17:15 Central European Time1.
The following affected States Parties participated in the information session of the meeting: Angola and the Democratic Republic of Congo.
The WHO Secretariat briefed the Committee on the history and impact of the Yellow Fever Initiative, the urban outbreak of yellow fever in Luanda, Angola and its national and international spread to the Democratic Republic of Congo, China and Kenya. The Committee was provided with additional information on the evolving risk of urban yellow fever in Africa and the status of the global stockpile of yellow fever vaccine.
After discussion and deliberation on the information provided, it was the decision of the Committee that the urban yellow fever outbreaks in Angola and the Democratic Republic of the Congo is a serious public health event which warrants intensified national action and enhanced international support. The Committee decided that based on the information provided the event does not at this time constitute a Public Health Emergency of International Concern (PHEIC).
While not considering the event currently to constitute a PHEIC, Members of the Committee strongly emphasized the serious national and international risks posed by urban yellow fever outbreaks and offered technical advice on immediate actions for the consideration of WHO and Member States in the following areas:
- the acceleration of surveillance, mass vaccination, risk communications, community mobilization, vector control and case management measures in Angola and the Democratic Republic of Congo;
- the assurance of yellow fever vaccination of all travellers, and especially migrant workers, to and from Angola and Democratic Republic of Congo;
- the intensification of surveillance and preparedness activities, including verification of yellow fever vaccination in travellers and risk communications, in at-risk countries and countries having land borders with the affected countries.
The Committee also emphasized the need to manage rapidly any new yellow fever importations, thoroughly evaluate ongoing response activities, and quickly expand yellow fever diagnostic and confirmatory capacity. Recognizing the limited international supply of yellow fever vaccines, the Committee also advised the immediate application of the policy of 1 lifetime dose of yellow fever vaccine2 and the rapid evaluation of yellow fever vaccine dose-sparing strategies by the WHO Strategic Advisory Group of Experts on Immunization (SAGE).
Going forward, the Committee agreed with the planned review and revision of the global strategy for preventing urban yellow fever outbreaks in keeping with WHO’s assessment that the risk of such events is increasing.
Based on these views and the currently available information, the Director-General accepted the Committee’s assessment that the current yellow fever situation is serious and of great concern and requires intensified control measures, but does not constitute a PHEIC at this time.
The Director-General urges Member States to enforce the yellow fever vaccination requirement for travellers to and from Angola and the Democratic Republic of the Congo in accordance with the IHR (2005)3
The Director-General thanked the Committee for its thorough advice on priority actions for affected and at-risk countries, and on further yellow fever risk management work for WHO. The Director-General appreciated the concurrence of the Committee to be reconvened if needed.
[1] The names and summary biographies of the Emergency Committee Members and Advisors are available at http://www.who.int/ihr/procedures/yellow-fever-ec-members/en/
[2] World Health Assembly Resolution WHA 67.13.
[3] as per Annex 7 of the International Health Regulations (2005)
Venezuela: In hospitals, supplies are lacking, electricity goes out, equipment is broken and patients lie in pools of blood.
Monday, May 16th, 2016How did a “training device” used in an exercise cause a Premier League match at Old Trafford to be postponed.
Monday, May 16th, 2016‘…..Assistant chief constable John O’Hare said: “I am grateful to the Manchester United and Bournemouth supporters for their support and assistance today.
“Following today’s controlled explosion, we have since found out that the item was a training device which had accidentally been left by a private company following a training exercise involving explosive search dogs.
“While this item did not turn out to be a viable explosive, on appearance this device was as real as could be, and the decision to evacuate the stadium was the right thing to do, until we could be sure that people were not at risk.”….’
WHO: Malaria Fact Sheet
Monday, May 16th, 2016Malaria
Key facts
- Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes.
- In 2015, 95 countries and territories had ongoing malaria transmission,
- About 3.2 billion people – almost half of the world’s population – are at risk of malaria.
- Malaria is preventable and curable, and increased efforts are dramatically reducing the malaria burden in many places.
- Between 2000 and 2015, malaria incidence among populations at risk (the rate of new cases) fell by 37% globally. In that same period, malaria death rates among populations at risk fell by 60% globally among all age groups, and by 65% among children under 5.
- Sub-Saharan Africa carries a disproportionately high share of the global malaria burden. In 2015, the region was home to 88% of malaria cases and 90% of malaria deaths.
Malaria is caused by Plasmodium parasites. The parasites are spread to people through the bites of infected female Anopheles mosquitoes, called “malaria vectors.” There are 5 parasite species that cause malaria in humans, and 2 of these species – P. falciparum and P. vivax – pose the greatest threat.
- P. falciparum is the most prevalent malaria parasite on the African continent. It is responsible for most malaria-related deaths globally.
- P. vivax is the dominant malaria parasite in most countries outside of sub-Saharan Africa.
Symptoms
Malaria is an acute febrile illness. In a non-immune individual, symptoms appear 7 days or more (usually 10–15 days) after the infective mosquito bite. The first symptoms – fever, headache, chills and vomiting – may be mild and difficult to recognize as malaria. If not treated within 24 hours, P. falciparum malaria can progress to severe illness, often leading to death.
Children with severe malaria frequently develop one or more of the following symptoms: severe anaemia, respiratory distress in relation to metabolic acidosis, or cerebral malaria. In adults, multi-organ involvement is also frequent. In malaria endemic areas, people may develop partial immunity, allowing asymptomatic infections to occur.
Who is at risk?
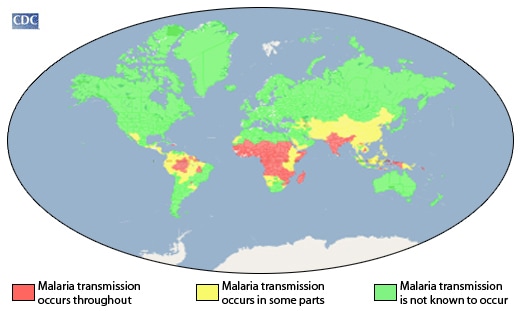
In 2015, approximately 3.2 billion people – nearly half of the world’s population – were at risk of malaria. Most malaria cases and deaths occur in sub-Saharan Africa. However, Asia, Latin America, and, to a lesser extent, the Middle East, are also at risk. In 2015, 95 countries and territories had ongoing malaria transmission.
Some population groups are at considerably higher risk of contracting malaria, and developing severe disease, than others. These include infants, children under 5 years of age, pregnant women and patients with HIV/AIDS, as well as non-immune migrants, mobile populations and travellers. National malaria control programmes need to take special measures to protect these population groups from malaria infection, taking into consideration their specific circumstances.
Disease burden
According to the latest WHO estimates, released in December 2015, there were 214 million cases of malaria in 2015 and 438 000 deaths.
Between 2000 and 2015, malaria incidence among populations at risk fell by 37% globally; during the same period, malaria mortality rates among populations at risk decreased by 60%. An estimated 6.2 million malaria deaths have been averted globally since 2001.
Sub-Saharan Africa continues to carry a disproportionately high share of the global malaria burden. In 2015, the region was home to 88% of malaria cases and 90% of malaria deaths.
Some 15 countries – mainly in sub-Saharan Africa – account for 80% of malaria cases and 78% deaths globally. Since 2000, the decline in malaria incidence in these 15 countries (32%) has lagged behind that of other countries globally (53%).
In areas with high transmission of malaria, children under 5 are particularly susceptible to infection, illness and death; more than two thirds (70%) of all malaria deaths occur in this age group. Between 2000 and 2015, the under-5 malaria death rate fell by 65% globally, translating into an estimated 5.9 million child lives saved between 2001 and 2015.
Transmission
In most cases, malaria is transmitted through the bites of female Anopheles mosquitoes. There are more than 400 different species of Anopheles mosquito; around 30 are malaria vectors of major importance. All of the important vector species bite between dusk and dawn. The intensity of transmission depends on factors related to the parasite, the vector, the human host, and the environment.
Anopheles mosquitoes lay their eggs in water, which hatch into larvae, eventually emerging as adult mosquitoes. The female mosquitoes seek a blood meal to nurture their eggs. Each species of Anopheles mosquito has its own preferred aquatic habitat; for example, some prefer small, shallow collections of fresh water, such as puddles and hoof prints, which are abundant during the rainy season in tropical countries.
Transmission is more intense in places where the mosquito lifespan is longer (so that the parasite has time to complete its development inside the mosquito) and where it prefers to bite humans rather than other animals. The long lifespan and strong human-biting habit of the African vector species is the main reason why nearly 90% of the world’s malaria cases are in Africa.
Transmission also depends on climatic conditions that may affect the number and survival of mosquitoes, such as rainfall patterns, temperature and humidity. In many places, transmission is seasonal, with the peak during and just after the rainy season. Malaria epidemics can occur when climate and other conditions suddenly favour transmission in areas where people have little or no immunity to malaria. They can also occur when people with low immunity move into areas with intense malaria transmission, for instance to find work, or as refugees.
Human immunity is another important factor, especially among adults in areas of moderate or intense transmission conditions. Partial immunity is developed over years of exposure, and while it never provides complete protection, it does reduce the risk that malaria infection will cause severe disease. For this reason, most malaria deaths in Africa occur in young children, whereas in areas with less transmission and low immunity, all age groups are at risk.
Prevention
Vector control is the main way to prevent and reduce malaria transmission. If coverage of vector control interventions within a specific area is high enough, then a measure of protection will be conferred across the community.
WHO recommends protection for all people at risk of malaria with effective malaria vector control. Two forms of vector control – insecticide-treated mosquito nets and indoor residual spraying – are effective in a wide range of circumstances.
Insecticide-treated mosquito nets
Long-lasting insecticidal nets (LLINs) are the preferred form of insecticide-treated mosquito nets (ITNs) for public health programmes. In most settings, WHO recommends LLIN coverage for all people at risk of malaria. The most cost-effective way to achieve this is by providing LLINs free of charge, to ensure equal access for all. In parallel, effective behaviour change communication strategies are required to ensure that all people at risk of malaria sleep under a LLIN every night, and that the net is properly maintained.
Indoor spraying with residual insecticides
Indoor residual spraying (IRS) with insecticides is a powerful way to rapidly reduce malaria transmission. Its full potential is realized when at least 80% of houses in targeted areas are sprayed. Indoor spraying is effective for 3–6 months, depending on the insecticide formulation used and the type of surface on which it is sprayed. In some settings, multiple spray rounds are needed to protect the population for the entire malaria season.
Antimalarial drugs
Antimalarial medicines can also be used to prevent malaria. For travellers, malaria can be prevented through chemoprophylaxis, which suppresses the blood stage of malaria infections, thereby preventing malaria disease. For pregnant women living in moderate-to-high transmission areas, WHO recommends intermittent preventive treatment with sulfadoxine-pyrimethamine, at each scheduled antenatal visit after the first trimester. Similarly, for infants living in high-transmission areas of Africa, 3 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine are recommended, delivered alongside routine vaccinations.
In 2012, WHO recommended Seasonal Malaria Chemoprevention as an additional malaria prevention strategy for areas of the Sahel sub-Region of Africa. The strategy involves the administration of monthly courses of amodiaquine plus sulfadoxine-pyrimethamine to all children under 5 years of age during the high transmission season.
Insecticide resistance
Much of the success in controlling malaria is due to vector control. Vector control is highly dependent on the use of pyrethroids, which are the only class of insecticides currently recommended for ITNs or LLINs.
In recent years, mosquito resistance to pyrethroids has emerged in many countries. In some areas, resistance to all 4 classes of insecticides used for public health has been detected. Fortunately, this resistance has only rarely been associated with decreased efficacy of LLINs, which continue to provide a substantial level of protection in most settings. Rotational use of different classes of insecticides for IRS is recommended as one approach to manage insecticide resistance.
However, malaria-endemic areas of sub-Saharan Africa and India are causing significant concern due to high levels of malaria transmission and widespread reports of insecticide resistance. The use of 2 different insecticides in a mosquito net offers an opportunity to mitigate the risk of the development and spread of insecticide resistance; developing these new nets is a priority. Several promising products for both IRS and nets are in the pipeline.
Detection of insecticide resistance should be an essential component of all national malaria control efforts to ensure that the most effective vector control methods are being used. The choice of insecticide for IRS should always be informed by recent, local data on the susceptibility of target vectors.
To ensure a timely and coordinated global response to the threat of insecticide resistance, WHO worked with a wide range of stakeholders to develop the “Global Plan for Insecticide Resistance Management in Malaria Vectors (GPIRM)“, which was released in May 2012.
Diagnosis and treatment
Early diagnosis and treatment of malaria reduces disease and prevents deaths. It also contributes to reducing malaria transmission. The best available treatment, particularly for P. falciparum malaria, is artemisinin-based combination therapy (ACT).
WHO recommends that all cases of suspected malaria be confirmed using parasite-based diagnostic testing (either microscopy or rapid diagnostic test) before administering treatment. Results of parasitological confirmation can be available in 30 minutes or less. Treatment, solely on the basis of symptoms should only be considered when a parasitological diagnosis is not possible. More detailed recommendations are available in the “WHO Guidelines for the treatment of malaria”, third edition, published in April 2015.
Antimalarial drug resistance
Resistance to antimalarial medicines is a recurring problem. Resistance of P. falciparum to previous generations of medicines, such as chloroquine and sulfadoxine-pyrimethamine (SP), became widespread in the 1970s and 1980s, undermining malaria control efforts and reversing gains in child survival.
WHO recommends the routine monitoring of antimalarial drug resistance, and supports countries to strengthen their efforts in this important area of work.
An ACT contains both the drug artemisinin and a partner drug. In recent years, parasite resistance to artemisinins has been detected in 5 countries of the Greater Mekong subregion: Cambodia, Lao People’s Democratic Republic, Myanmar, Thailand and Viet Nam. Studies have confirmed that artemisinin resistance has emerged independently in many areas of this subregion. Most patients are cured when treated with an ACT if there is no resistance to the partner drug.
However, in parts of Cambodia and Thailand, P. falciparum resistance to both artemisinin and partner drugs (multi-drug resistance) has developed.
There are concerns that P. falciparum malaria in Cambodia and Thailand is becoming increasingly difficult to treat, and that multi-drug resistance could spread to other regions with dire public health consequences. Consequently, WHO’s Malaria Policy Advisory Committee in September 2014 recommended adopting the goal of eliminating P. falciparum malaria in this subregion by 2030. WHO launched the Strategy for Malaria Elimination in the Greater Mekong Subregion (2015–2030) at the World Health Assembly in May 2015, which was endorsed by all the countries in the subregion.
Surveillance
Surveillance entails tracking of the disease and programmatic responses, and taking action based on the data received. Currently many countries with a high burden of malaria have weak surveillance systems and are not in a position to assess disease distribution and trends, making it difficult to optimize responses and respond to outbreaks.
Effective surveillance is required at all points on the path to malaria elimination. Strong malaria surveillance enables programmes to optimize their operations, by empowering programmes to:
- advocate for investment from domestic and international sources, commensurate with the malaria disease burden in a country or subnational area;
- allocate resources to populations most in need and to interventions that are most effective, in order to achieve the greatest possible public health impact;
- assess regularly whether plans are progressing as expected or whether adjustments in the scale or combination of interventions are required;
- account for the impact of funding received and enable the public, their elected representatives and donors to determine if they are obtaining value for money; and
- evaluate whether programme objectives have been met and learn what works so that more efficient and effective programmes can be designed.
Stronger malaria surveillance systems are urgently needed to enable a timely and effective malaria response in endemic regions, to prevent outbreaks and resurgences, to track progress, and to hold governments and the global malaria community accountable.
Elimination
Malaria elimination is defined as the interruption of local transmission of a specified malaria parasite in a defined geographical area as a result of deliberate efforts. Continued measures are required to prevent re-establishment of transmission.
Malaria eradication is defined as the permanent reduction to 0 of the worldwide incidence of malaria infection caused by human malaria parasites as a result of deliberate efforts. Once eradication has been achieved, intervention measures are no longer needed.
The rate of progress in a particular country will depend on the strength of its national health system, the level of investment in malaria control, and a number of other factors, including: biological determinants, the environment, and the social, demographic, political, and economic realities of a particular country.
In countries with high or moderate rates of malaria transmission, national malaria control programmes aim to maximize the reduction of malaria cases and deaths.
As countries approach elimination, enhanced surveillance systems can help ensure that every infection is detected, treated and reported to a national malaria registry. Patients diagnosed with malaria should be treated promptly with effective antimalarial medicines for their own health and to prevent onward transmission of the disease in the community.
Countries that have achieved at least 3 consecutive years of 0 local cases of malaria are eligible to apply for the WHO certification of malaria elimination. In recent years, 5 countries have been certified by the WHO Director-General as having eliminated malaria: United Arab Emirates (2007), Morocco (2010), Turkmenistan (2010), Armenia (2011) and Maldives (2015). Recently 3 other countries started the certification process: Argentina, Kyrgyzstan and Sri Lanka.
Vaccines against malaria
There are currently no licensed vaccines against malaria or any other human parasite. One research vaccine against P. falciparum, known as RTS, S/AS01, is most advanced. This vaccine has been evaluated in a large clinical trial in 7 countries in Africa and received a positive opinion by the European Medicines Agency in July 2015.
In October 2015, 2 WHO advisory groups recommended pilot implementations of RTS, S/AS01 in a limited number of African countries. WHO has adopted these recommendations and is strongly supportive of the need to proceed with these pilots as the next step for the world’s first malaria vaccine. These pilot projects could pave the way for wider deployment of the vaccine in 3 to 5 years, if safety and effectiveness are considered acceptable.
WHO response
The WHO Global Technical Strategy for Malaria 2016-2030 – adopted by the World Health Assembly in May 2015 – provides a technical framework for all malaria-endemic countries. It is intended to guide and support regional and country programmes as they work towards malaria control and elimination.
The Strategy sets ambitious but achievable global targets, including:
- Reducing malaria case incidence by at least 90% by 2030.
- Reducing malaria mortality rates by at least 90% by 2030.
- Eliminating malaria in at least 35 countries by 2030.
- Preventing a resurgence of malaria in all countries that are malaria-free.
This Strategy was the result of an extensive consultative process that spanned 2 years and involved the participation of more than 400 technical experts from 70 Member States. It is based on 3 key pillars:
- ensuring universal access to malaria prevention, diagnosis and treatment;
- accelerating efforts towards elimination and attainment of malaria-free status; and
- transforming malaria surveillance into a core intervention.
The WHO Global Malaria Programme (GMP) coordinates WHO’s global efforts to control and eliminate malaria by:
- setting, communicating and promoting the adoption of evidence-based norms, standards, policies, technical strategies, and guidelines;
- keeping independent score of global progress;
- developing approaches for capacity building, systems strengthening, and surveillance; and
- identifying threats to malaria control and elimination as well as new areas for action.
GMP is supported and advised by the Malaria Policy Advisory Committee (MPAC), a group of 15 global malaria experts appointed following an open nomination process. The MPAC, which meets twice yearly, provides independent advice to WHO to develop policy recommendations for the control and elimination of malaria. The mandate of MPAC is to provide strategic advice and technical input, and extends to all aspects of malaria control and elimination, as part of a transparent, responsive and credible policy setting process.
U.K.: The Premier League match between United and Bournemouth was called off after the stadium, which has a capacity for more than 75,000 fans, was evacuated because a suspicious package was found of
Sunday, May 15th, 2016SL-LPA: A rapid diagnostic test (24-48 hrs.) for identifying those MDR- or rifampicin-resistant TB patients
Sunday, May 15th, 2016Factsheet_MDR-TB-Quik-Assay-2016
This test is the first and only WHO-recommended rapid test for detection of additional resistance in MDR-TB patients as well as XDR-TB.

Rapid diagnostic test and shorter, cheaper treatment signal new hope for multidrug-resistant tuberculosis patients
Sunday, May 15th, 2016TB_Short_MDR_regimen_factsheet-WHO
12 MAY 2016 | GENEVA – New WHO recommendations aim to speed up detection and improve treatment outcomes for multidrug resistant tuberculosis (MDR-TB) through use of a novel rapid diagnostic test and a shorter, cheaper treatment regimen.
“This is a critical step forward in tackling the MDR-TB public health crisis,” said Dr Mario Raviglione, Director of WHO’s Global TB Programme. “The new WHO recommendations offer hope to hundreds of thousands of MDR-TB patients who can now benefit from a test that quickly identifies eligibility for the shorter regimen, and then complete treatment in half the time and at nearly half the cost.”
Shorter treatment with better outcomes
At less than US$ 1000 per patient, the new treatment regimen can be completed in 9–12 months. Not only is it less expensive than current regimens, but it is also expected to improve outcomes and potentially decrease deaths due to better adherence to treatment and reduced loss to follow-up.
The conventional treatment regimens, which take 18–24 months to complete, yield low cure rates: just 50% on average globally. This is largely because patients find it very hard to keep taking second-line drugs, which can be quite toxic, for prolonged periods of time. They therefore often interrupt treatment or are lost to follow-up in health services.
The shorter regimen is recommended for patients diagnosed with uncomplicated MDR-TB, for example those individuals whose MDR-TB is not resistant to the most important drugs used to treat MDR-TB (fluoroquinolones and injectables), known as “second-line drugs”. It is also recommended for individuals who have not yet been treated with second line drugs.
WHO’s recommendations on the shorter regimens are based on initial programmatic studies involving 1200 patients with uncomplicated MDR-TB in 10 countries . WHO is urging researchers to complete ongoing randomised controlled clinical trials in order to strengthen the evidence base for use of this regimen.
Rapid diagnostic test to identify second-line drug resistance
The most reliable way to rule out resistance to second-line drugs is a newly recommended diagnostic test for use in national TB reference laboratories. The novel diagnostic test – called MTBDRsl – is a DNA-based test that identifies genetic mutations in MDR-TB strains, making them resistant to fluoroquinolones and injectable second-line TB drugs.
This test yields results in just 24-48 hours, down from the 3 months or longer currently required. The much faster turnaround time means that MDR-TB patients with additional resistance are not only diagnosed more quickly, but can quickly be placed on appropriate second-line regimens. WHO reports that fewer than 20% of the estimated 480 000 MDR-TB patients globally are currently being properly treated.
The MTBDRsl test is also a critical prerequisite for identifying MDR-TB patients who are eligible for the newly recommended shorter regimen, while avoiding placing patients who have resistance to second-line drugs on this regimen (which could fuel the development of extensively drug-resistant TB or XDR-TB).
“We hope that the faster diagnosis and shorter treatment will accelerate the much-needed global MDR-TB response,” said Dr Karin Weyer, Coordinator of Laboratories, Diagnostics and Drug Resistance, WHO Global TB Programme. “Anticipated cost-savings from the roll out of this regimen could be re-invested in MDR-TB services to enable more patients to be tested and retained on treatment.”
WHO is working closely with technical and funding partners to ensure adequate resources and support for the uptake of the rapid test and shorter, cheaper regimen in countries.
Quick facts
- Resistance to standard TB drugs exists in most countries worldwide. Drug resistance, fuelled by inadequate treatment, can spread through the air, from person to person, in the same way as drug-susceptible TB.
- Multidrug-resistant TB (MDR-TB) is caused by TB bacteria that are resistant to at least isoniazid and rifampicin, the two most effective TB drugs. Based on figures from 2014, the latest year for which data are available, WHO estimates that 5% of TB cases are multidrug-resistant. This translates into 480 000 cases and 190 000 deaths each year.
- Extensively drug-resistant TB (XDR-TB) is a form of MDR-TB that is also resistant to any fluoroquinolone and any of the second–line anti-TB injectable agents (i.e. amikacin, kanamycin or capreomycin). About 9% of MDR-TB patients develop XDR-TB, which is even more difficult to treat.
- The WHO “End TB Strategy“, adopted by all WHO Member States, serves as a blueprint for countries to reduce TB incidence by 80% and TB deaths by 90%, and to eliminate catastrophic costs for TB-affected households by 2030.
A charter bus heading to a casino crashed in far South Texas on Saturday, killing 8 and injuring 44 others in a one-vehicle rollover
Sunday, May 15th, 2016Learn about Public Safety Canada
Sunday, May 15th, 2016Learn about how Public Safety Canada engages at all levels during a crisis to ensure the best outcome for Canadians.
Responding to emergency events
If an emergency escalates beyond the capabilities of the provincial or territorial government, assistance may be provided by the federal government. Find out more about the process and the types of support that could be offered.
Government Operations Centre
The Government Operations Centre (GOC), housed at Public Safety Canada, leads and coordinates the federal response during a crisis on behalf of the Government of Canada.
Provincial and Territorial emergency management organizations
Provincial and territorial emergency management organizations (EMOs) are a good source of information about how to prepare for emergencies in your region.
Disaster Financial Assistance Arrangements
The Government of Canada provides cost-shared financial assistance, administered by Public Safety Canada, to provincial and territorial governments to help with extraordinary costs incurred by large scale natural disasters.
Get Prepared
All Canadians have a role in building resilient communities, helping to keep hazards from becoming disasters, and in recovering from disasters when they do happen. Find out how to prepare for risks in your region.
Acts and Regulations
- Emergency Management Act
- Emergency Management Framework for Canada
- Federal Policy for Emergency Management
- Date modified:
- 2016-05-06
Puerto Rico: First American whose fetus developed microcephaly because of Zika virus
Saturday, May 14th, 2016CDC has estimated that 20 percent or more of the island’s 3.5 million residents will become infected with Zika this year.





