Archive for the ‘Influenza’ Category
2018-2019 U.S. Flu Season: Preliminary Burden Estimates from October 1, 2018, through February 16, 2019
Saturday, February 23rd, 2019CCDC estimates that, from , there have been:
CDC
* 17.7 million – 20.4 million flu illnesses
![]()
- 8.2 million – 9.6 million flu medical visits
- 214,000 – 256,000 flu hospitalizations
- 13,600 – 22,300 flu deaths
This season’s flu vaccine efficacy: “….the overall estimated effectiveness of seasonal influenza vaccine for preventing medically attended, laboratory-confirmed influenza virus infection was 47%…”
Monday, February 18th, 2019“…..On the basis of data from the U.S. Influenza Vaccine Effectiveness Network on 3,254 children and adults with acute respiratory illness during November 23, 2018–February 2, 2019, the overall estimated effectiveness of seasonal influenza vaccine for preventing medically attended, laboratory-confirmed influenza virus infection was 47%.….”
Doyle JD, Chung JR, Kim SS, et al. Interim Estimates of 2018–19 Seasonal Influenza Vaccine Effectiveness — United States, February 2019. MMWR Morb Mortal Wkly Rep 2019;68:135–139. DOI: http://dx.doi.org/10.15585/mmwr.mm6806a2.
Flu Activity in the U.S. up to February 2, 2019: Maybe it isn’t as bad as we are led to believe!
Monday, February 18th, 2019“……Influenza activity in the United States remained elevated through February 2, 2019, and is expected to continue for several more weeks. Compared with recent influenza seasons, as of February 2, 2019, severity this season has been low, with a lower percentage of outpatient visits for influenza-like illness, lower rates of hospitalization, and fewer deaths attributed to pneumonia and influenza…..”
Blanton L, Dugan VG, Abd Elal AI, et al. Update: Influenza Activity — United States, September 30, 2018–February 2, 2019. MMWR Morb Mortal Wkly Rep 2019;68:125–134. DOI: http://dx.doi.org/10.15585/mmwr.mm6806a1.
2018-2019 Influenza Season Week 6 ending February 9, 2019: Influenza activity continues to increase in the United States.
Saturday, February 16th, 2019Latest research: “End-of-season influenza vaccine efficacy for the 2017-2018 flu season was a mediocre 38% (95% CI 31%-43%), but flu shots were still estimated to have prevented 7 million illnesses and 8,000 deaths…”
Monday, February 11th, 2019Effects of Influenza Vaccination in the United States during the 2017–2018 Influenza Season
2018-2019 Influenza Season Week 5 ending February 2, 2019: Influenza activity increased in the United States.
Saturday, February 9th, 2019Influenza activity increased in the United States. Influenza A(H1N1)pdm09, influenza A(H3N2), and influenza B viruses continue to co-circulate. Below is a summary of the key influenza indicators for the week ending February 2, 2019
- Viral Surveillance:The percentage of respiratory specimens testing positive for influenza viruses in clinical laboratories increased. Influenza A viruses have predominated in the United States since the beginning of October. Influenza A(H1N1)pdm09 viruses have predominated in most areas of the country, however influenza A(H3) viruses have predominated in the southeastern United States (HHS Region 4).
- Virus Characterization:The majority of influenza viruses characterized antigenically and genetically are similar to the cell-grown reference viruses representing the 2018–2019 Northern Hemisphere influenza vaccine viruses.
- Antiviral Resistance:The vast majority of influenza viruses tested (>99%) show susceptibility to oseltamivir and peramivir. All influenza viruses tested showed susceptibility to zanamivir.
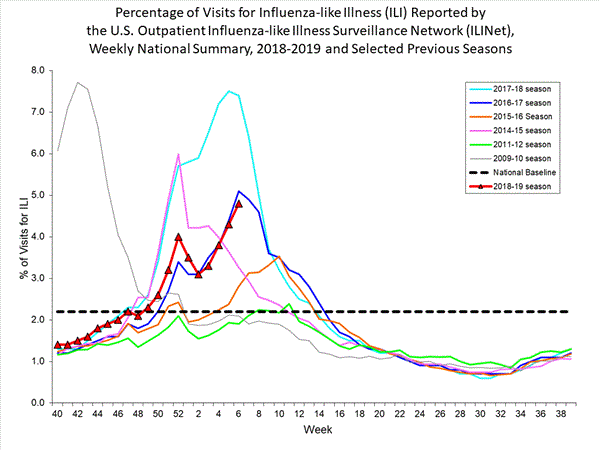
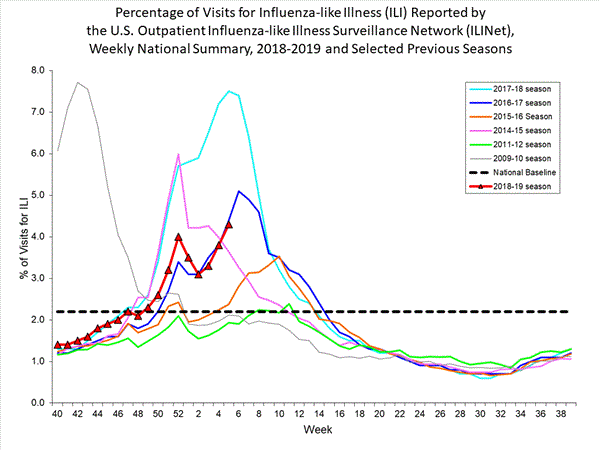
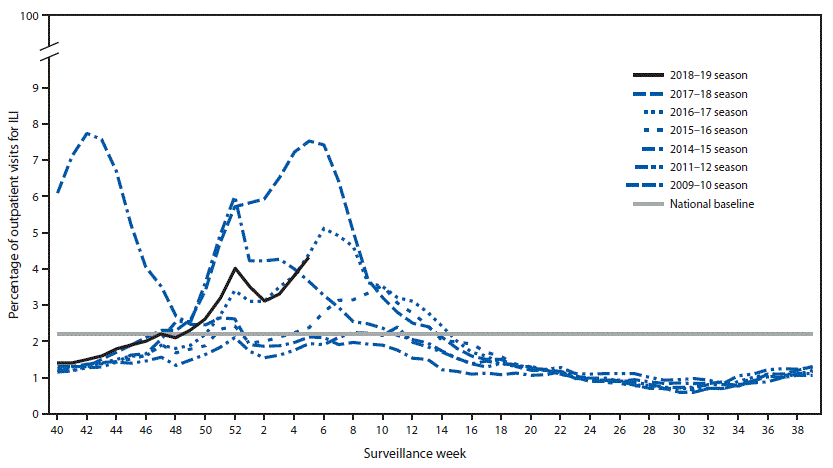
- Influenza-like Illness Surveillance:The proportion of outpatient visits for influenza-like illness (ILI) increased to 4.3%, which is above the national baseline of 2.2%. All 10 regions reported ILI at or above their region-specific baseline level.
- ILI State Activity Indictor Map: New York City and 24 states experienced high ILI activity; Puerto Rico and 10 states experienced moderate ILI activity; the District of Columbia and 13 states experienced low ILI activity; and three states experienced minimal ILI activity.
- Geographic Spread of Influenza: The geographic spread of influenza in Puerto Rico and 47 states was reported as widespread; two states reported regional activity; the District of Columbia and one state reported local activity; the U.S. Virgin Islands reported sporadic activity; and Guam did not report.
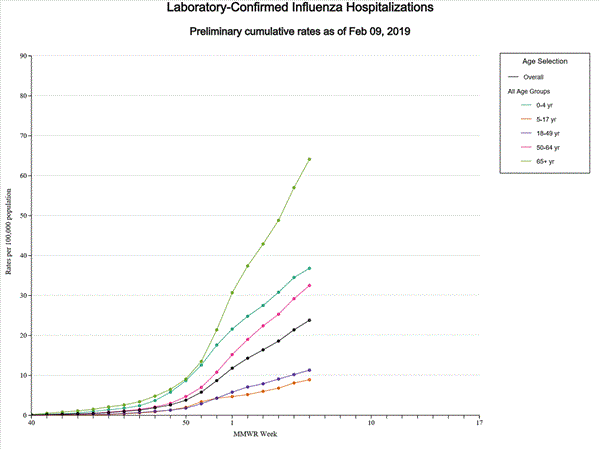
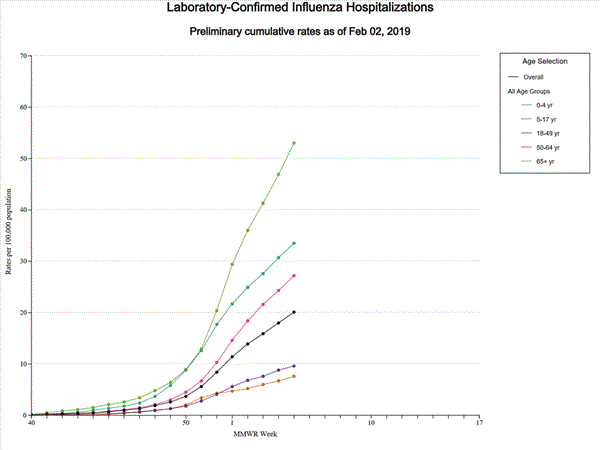
- Influenza-associated Hospitalizations A cumulative rate of 20.1 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported. The highest hospitalization rate is among adults 65 years and older (53.0 hospitalizations per 100,000 population).
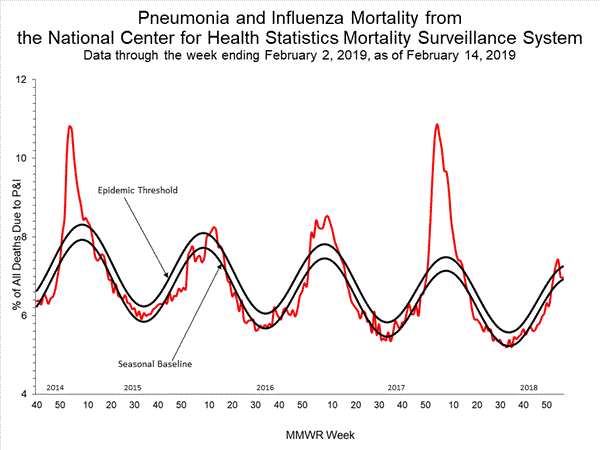
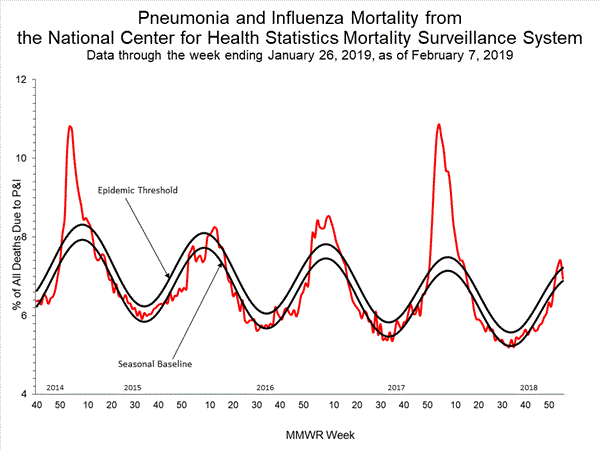
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the National Center for Health Statistics (NCHS) Mortality Surveillance System.
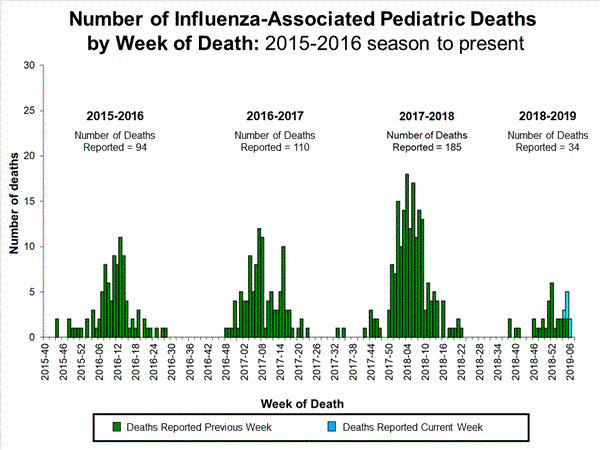
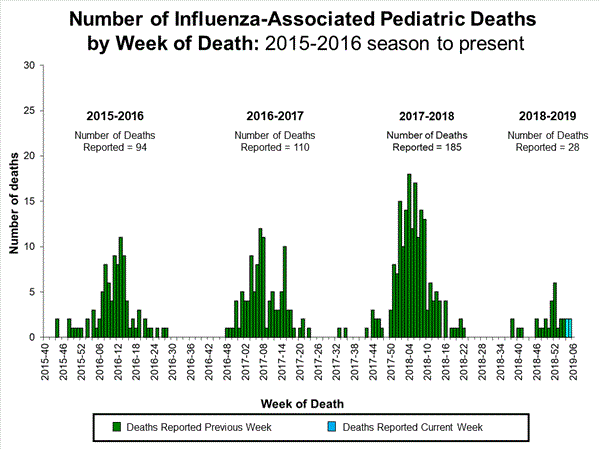
- Influenza-associated Pediatric Deaths: Four influenza-associated pediatric deaths were reported to CDC during week 5.
World influenza update as of 1 February 2019
Thursday, February 7th, 2019
Summary
In the temperate zone of the northern hemisphere influenza activity continued to increase.
- In North America, influenza activity appeared to decrease slightly with influenza A(H1N1)pdm09 predominating.
- In Europe, influenza activity continued to increase, with both A viruses circulating.
- In North Africa, influenza A(H1N1)pdm09 detections sharply increased in Morocco.
- In Western Asia, influenza activity continued to increase in some countries and appeared to decrease across countries of the Arabian Peninsula.
- In East Asia, influenza activity continued to increase, with influenza A(H1N1)pdm09 virus predominating.
- In Southern Asia, influenza detections remained elevated overall. Influenza activity continued to increase in Iran (Islamic Republic of) with influenza A(H3N2) the predominant circulating virus.
- In the temperate zones of the southern hemisphere, influenza activity remained at inter-seasonal levels.
- Worldwide, seasonal influenza A viruses accounted for the majority of detections.
National Influenza Centres (NICs) and other national influenza laboratories from 110 countries, areas or territories reported data to FluNet for the time period from 07 January 2019 to 20 January 2019 (data as of 2019-02-01 04:30:14 UTC). The WHO GISRS laboratories tested more than 232136 specimens during that time period. 59457 were positive for influenza viruses, of which 58436 (98.3%) were typed as influenza A and 1021 (1.7%) as influenza B. Of the sub-typed influenza A viruses, 24559 (77.7%) were influenza A(H1N1)pdm09 and 7058 (22.3%) were influenza A(H3N2). Of the characterized B viruses, 85 (34.6%) belonged to the B-Yamagata lineage and 161 (65.4%) to the B-Victoria lineage.
The Flu in Europe, Week 4 (21-27 January 2019): Influenza activity continued to increase, with both A viruses circulating.
Thursday, February 7th, 2019New research: Contamination of health care personnel during removal of contaminated gloves
Tuesday, February 5th, 2019
Alhmidi H, Gonzalez-Orta M, Cadnum JL, et al. Contamination of health care personnel during removal of contaminated gloves. Am J Infect Control 2019 (published online Jan 9)
“…..In simulations of contaminated glove removal, 37% of health care personnel using their typical doffing technique contaminated their skin with a fluorescent solution. The frequency of contamination was significantly lower when the technique recommended by the Centers for Disease Control and Prevention was used versus not used (8 of 34, 24% vs 29 of 66, 44%). In simulations in which only the palm of the glove was contaminated, a modified doffing technique, to minimize the risk for contact with contaminated surfaces, reduced contamination of personnel……”
Oxford University (Oxford University Innovation) has entered into an option agreement for a universal influenza vaccine with US-based startup Blue Water Vaccines (BWV)
Saturday, February 2nd, 2019“……there are over almost 500,000 deaths a year caused by influenza, with somewhere between three to five million severe cases of illness annually. The virus is estimated to cost $87.1 billion in absenteeism in the US alone...…
[T]he universal flu vaccine developed at Oxford University targets epitopes that are both naturally immunogenic and limited in variability, allowing for a vaccine that protects against all influenza strains and avoids the need to be regularly updated.
The vaccine, which covers all influenza A and B strains is, is approaching clinical studies and could feasibly enter Phase I trials by the end of 2019……….”
‘……The vaccine is designed to target components of the influenza virus that are common to all strains, and will therefore also be suitable to target the outbreak of a new flu pandemic caused by the emergence of a novel form of the virus at the time it moves from an animal species into humans, according to the firm.
The vaccine is 100 percent synthetic and delivers “highly conserved immunogenic peptide fragments from the flu virus to antigen presenting cells in the skin, eliciting a strong and long-lasting T-cell immune response”……’
“…..Unlike ordinary vaccines, which use proteins found on the surface of the virus that are susceptible to change, the universal vaccine uses the more stable proteins at the virus’ core. Instead of utilising antibodies, it works by stimulating the immune system to boost virus-killing T-cells, which research has shown such can help fight more than one type of flu virus...…”