Archive for the ‘Influenza’ Category
CDC Health Advisory: Flu Season Begins — Severe Influenza Illness Reported
Tuesday, February 2nd, 2016
|
||
Weekly U.S. Influenza Surveillance Report
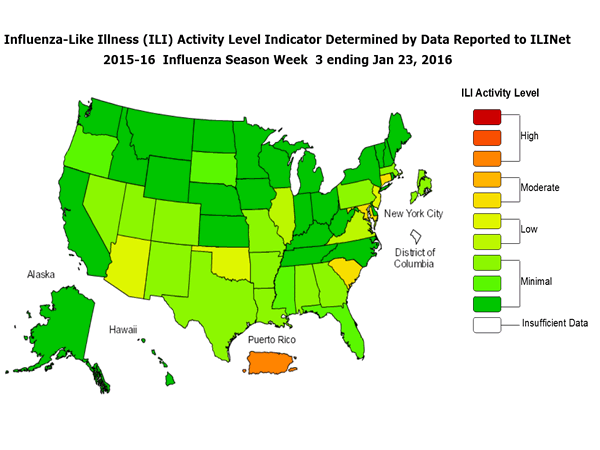
Sunday, January 31st, 2016During week 3 (January 17-23, 2016), influenza activity increased slightly in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 3 was influenza A, with influenza A (H1N1)pdm09 viruses predominating. The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
<!–
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
–>
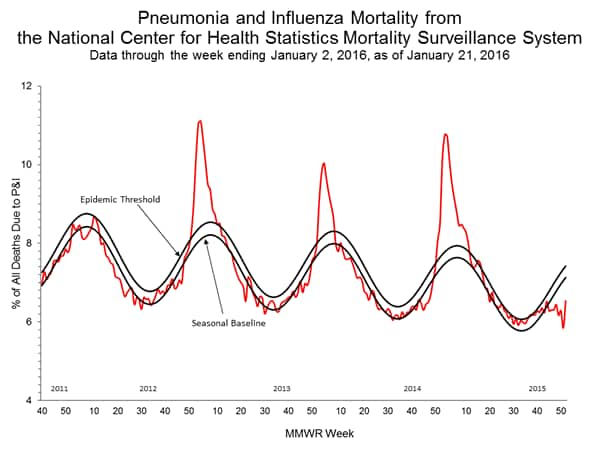
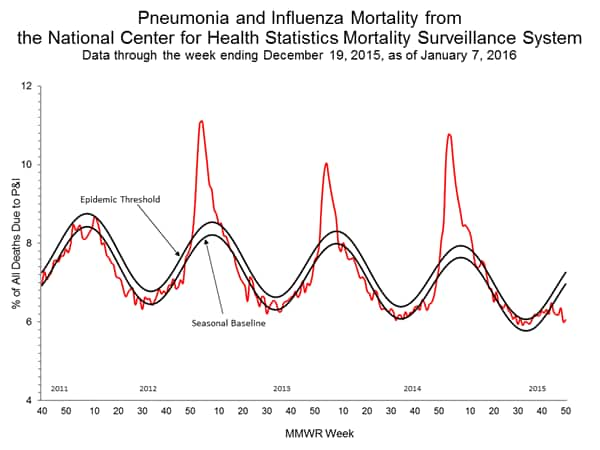
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below their system-specific epidemic threshold in both the NCHS Mortality Surveillance System and the 122 Cities Mortality Reporting System.
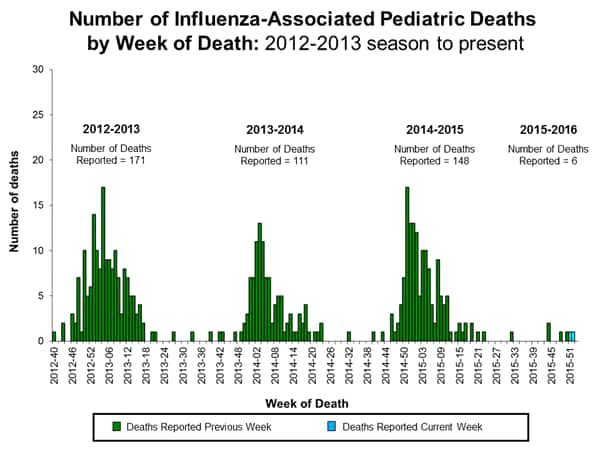
- Influenza-associated Pediatric Deaths: No influenza-associated pediatric deaths were reported.
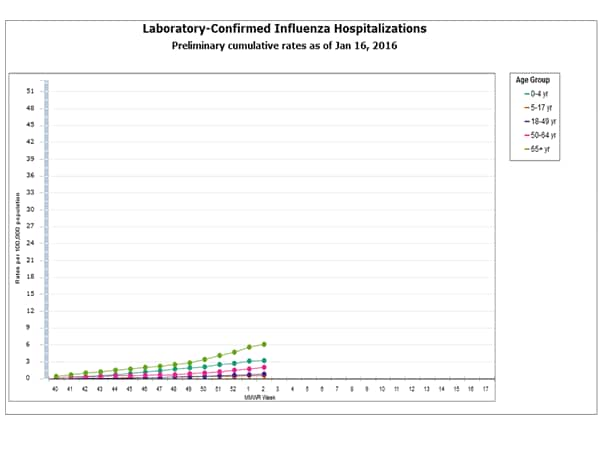
- Influenza-associated Hospitalizations: A cumulative rate for the season of 2.1 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
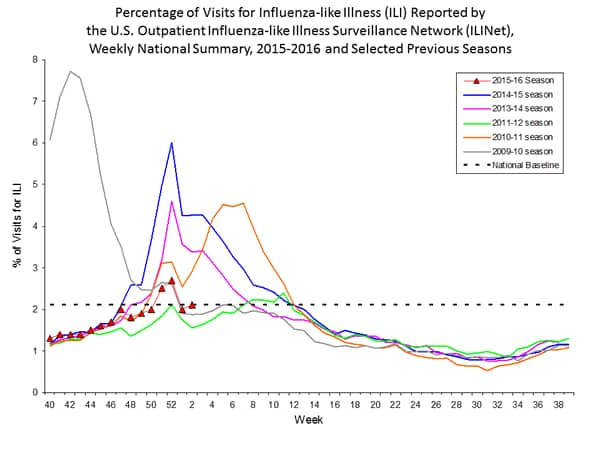
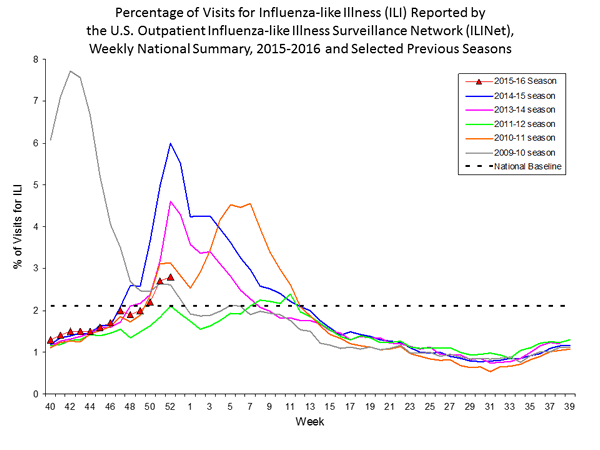
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.2%, which is above the national baseline of 2.1%. Six of 10 regions reported ILI at or above region-specific baseline levels. Puerto Rico experienced high ILI activity; three states experienced moderate ILI activity; five states experienced low ILI activity; New York City and 42 states experienced minimal ILI activity; and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in four states was reported as widespread; Puerto Rico and 14 states reported regional activity; Guam and 12 states reported local activity; and the District of Columbia, the U.S. Virgin Islands and 20 states reported sporadic activity.
2015-2016 Influenza Season Week 2 ending January 16, 2016
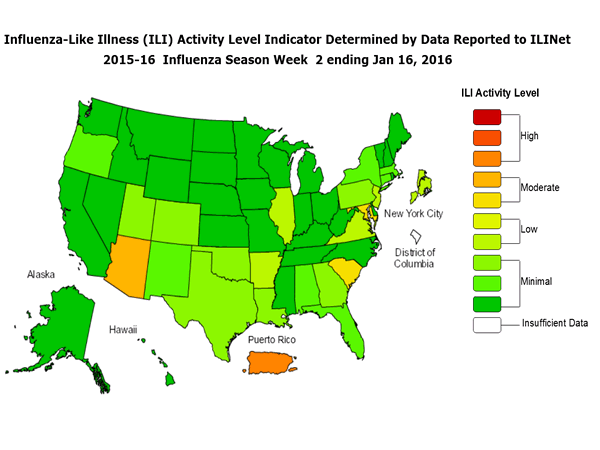
Sunday, January 24th, 2016During week 2 (January 10-16, 2016), influenza activity increased slightly in the United States.
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 2 was influenza A, with influenza A (H1N1)pdm09 viruses predominating. The percentage of respiratory specimens testing positive for influenza in clinical laboratories increased.
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
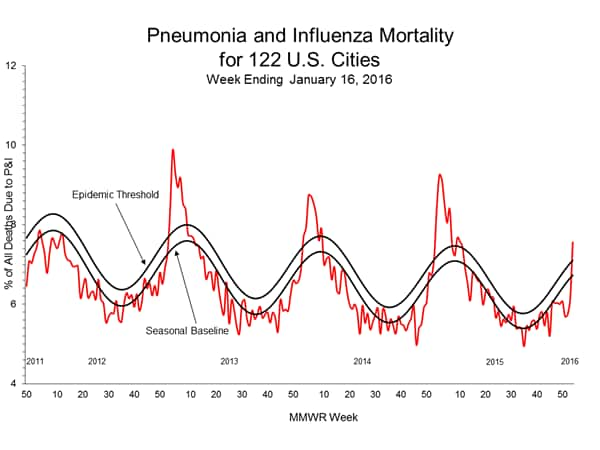
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below the system-specific epidemic threshold in the NCHS Mortality Surveillance System and above the system-specific epidemic threshold in the 122 Cities Mortality Reporting System.
- Influenza-associated Pediatric Deaths: No influenza-associated pediatric deaths were reported.
- Influenza-associated Hospitalizations: A cumulative rate for the season of 1.8 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.1%, which is at the national baseline of 2.1%. Six of 10 regions reported ILI at or above region-specific baseline levels. Puerto Rico experienced high ILI activity; three states experienced moderate ILI activity; New York City and four states experienced low ILI activity; 43 states experienced minimal ILI activity; and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in three states was reported as widespread; Puerto Rico and 10 states reported regional activity; Guam and 12 states reported local activity; the U.S. Virgin Islands and 24 states reported sporadic activity; and the District of Columbia and one state reported no influenza activity.
Region and state-specific data are available at http://www.cdc.gov/flu/weekly/nchs.htm.
CDC: 2015-2016 Influenza Season Week 1 ending January 9, 2016
Monday, January 18th, 2016Background:
The Centers for Disease Control and Prevention’s (CDC) Influenza Division collects, compiles, and analyzes information on influenza activity year-round in the United States and produces FluView, a weekly influenza surveillance report, and FluView Interactive. The U.S. influenza surveillance system provides information in five categories collected from nine data sources. This is the first report of the 2015-2016 influenza season, which began on October 4, 2015.
The five categories and nine data components of CDC influenza surveillance are:
- Viral Surveillance:U.S. World Health Organization (WHO) collaborating laboratories, the National Respiratory and Enteric Virus Surveillance System (NREVSS), and human infection with novel influenza A virus case reporting;
- Mortality:National Center for Health Statistics (NCHS) Mortality Surveillance System, 122 Cities Mortality Reporting System and influenza-associated pediatric deaths;
- Hospitalizations:Influenza Hospitalization Network (FluSurv-NET) including the Emerging Infections Program (EIP) and three additional states;
- Outpatient Illness Surveillance:U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet);
- Geographic Spread of Influenza:State and territorial epidemiologists’ reports.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.
–>
Synopsis:
During week 1 (January 3-9, 2016), laboratory data indicated that influenza activity increased slightly in the United States.
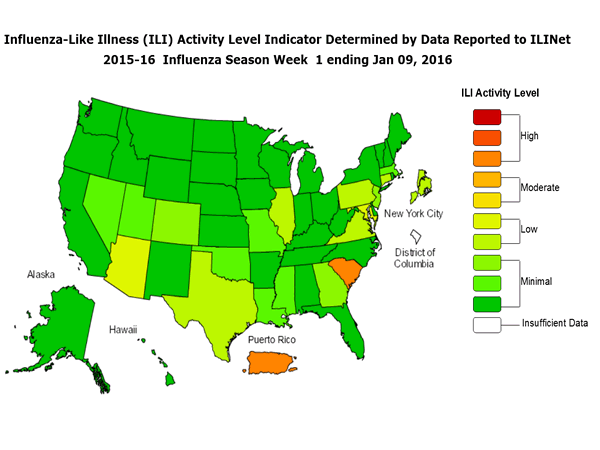
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 1 was influenza A, with influenza A (H1N1)pdm09 viruses predominating. The percentage of respiratory specimens testing positive for influenza in clinical laboratories was low.
<!–
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
–>
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below their system-specific epidemic threshold in both the NCHS Mortality Surveillance System and the 122 Cities Mortality Reporting System.
- Influenza-associated Pediatric Deaths: One influenza-associated pediatric death was reported.
- Influenza-associated Hospitalizations: A cumulative rate for the season of 1.5 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.0%, which is below the national baseline of 2.1%. Four of 10 regions reported ILI at or above region-specific baseline levels. Puerto Rico and one state experienced high ILI activity; New York City and seven states experienced low ILI activity; 42 states experienced minimal ILI activity; and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in Guam, Puerto Rico, and nine states were reported as regional; 11 states reported local activity; the U.S. Virgin Islands and 28 states reported sporadic activity; and the District of Columbia and two states reported no influenza activity.
Weekly U.S. Influenza Surveillance Report: 2015-2016 Influenza Season Week 52 ending January 2, 2016
Monday, January 11th, 2016Synopsis:
During week 52 (December 26, 2015-January 2, 2016), influenza activity increased slightly in the United States.
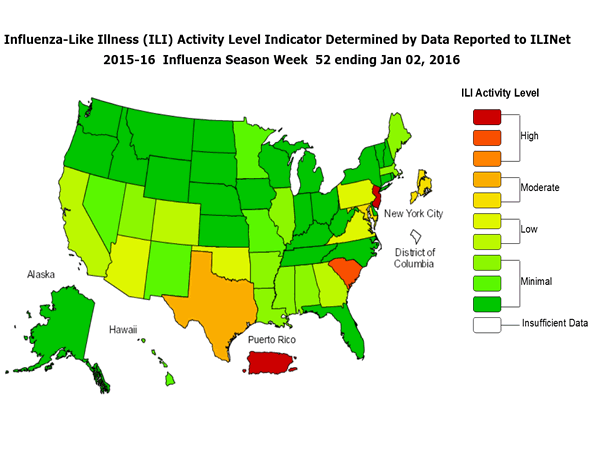
- Viral Surveillance: The most frequently identified influenza virus type reported by public health laboratories during week 52 was influenza A, with influenza A (H1N1)pdm09 viruses predominating. The percentage of respiratory specimens testing positive for influenza in clinical laboratories was low.
- Novel Influenza A Virus: One human infection with a novel influenza A virus was reported.
- Pneumonia and Influenza Mortality: The proportion of deaths attributed to pneumonia and influenza (P&I) was below their system-specific epidemic threshold in both the NCHS Mortality Surveillance System and the 122 Cities Mortality Reporting System.
- Influenza-associated Pediatric Deaths: Two influenza-associated pediatric deaths were reported.
<!–
- Influenza-associated Hospitalizations: A cumulative rate for the season of 65.5 laboratory-confirmed influenza-associated hospitalizations per 100,000 population was reported.
–>
- Outpatient Illness Surveillance: The proportion of outpatient visits for influenza-like illness (ILI) was 2.8%, which is above the national baseline of 2.1%. Seven of 10 regions reported ILI at or above region-specific baseline levels. Puerto Rico and two states experienced high ILI activity; New York City and two states experienced moderate ILI activity; seven states experienced low ILI activity; 39 states experienced minimal ILI activity; and the District of Columbia had insufficient data.
- Geographic Spread of Influenza: The geographic spread of influenza in Guam and two states were reported as widespread; six states reported regional activity; 13 states reported local activity; the U.S. Virgin Islands and 27 states reported sporadic activity; the District of Columbia and two states reported no influenza activity; and Puerto Rico did not report.
An estimated 43,900 excess winter deaths occurred in England and Wales in 2014/15.
Thursday, November 26th, 2015UK Office for National Statistics
An estimated 43,900 excess winter deaths occurred in England and Wales in 2014/15; the highest number since 1999/00, with 27% more people dying in the winter months compared with the non-winter months.
- The majority of deaths occurred among people aged 75 and over; there were an estimated 36,300 excess winter deaths in this age group in 2014/15, compared with 7,700 in people aged under 75.
- There were more excess winter deaths in females than in males in 2014/15, as in previous years. Male excess winter deaths increased from 7,210 to 18,400, and female deaths from 10,250 to 25,500 between 2013/14 and 2014/15.
- Respiratory diseases were the underlying cause of death in more than a third of all excess winter deaths in 2014/15.
- The excess winter mortality index was highest in the South West in 2014/15 and joint lowest in Yorkshire and The Humber, and Wales.